Abstract
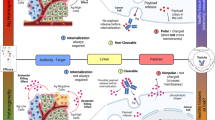
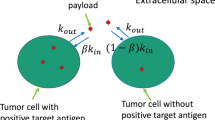
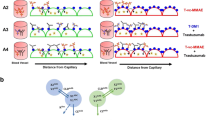
Antibody–drug conjugates (ADCs) are designed to target antigen expressing (Ag+) cells in a tumor. Once processed by the Ag+ cells, ADCs can release cytotoxic drug molecules that can diffuse out of Ag+ cells into the neighboring antigen-negative (Ag−) cells to induce their cytotoxicity. This additional efficacy of ADCs on Ag− cells in the presence of Ag+ cells is known as the ‘bystander effect’. Although the importance of this phenomena is widely acknowledged for effective killing of a heterogeneous tumor, the rate and extent of the bystander killing in a heterogeneous system is not quantitatively understood yet. Thus, the objectives of this manuscript were to: (1) synthesize and characterize a tool ADC Trastuzumab-vc-MMAE that is capable of exhibiting bystander effect, (2) quantify the time course of the bystander effect for the tool ADC using in vitro co-culture systems created using mixture of various HER2-expressing cell lines, and (3) develop a pharmacodynamic (PD) model that is capable of characterizing the bystander effect of ADCs. Co-culture studies conducted using GFP labelled MCF7 cells as Ag− cells and N87, BT474, and SKBR3 as Ag+ cells revealed that the bystander effect of ADC increases with increasing fraction of Ag+ cells in a co-culture system, and with increased expression level of target on Ag+ cells. A notable lag time after ADC incubation was also observed prior to significant bystander killing of Ag− cells. Based on our results we hypothesize that there may be other determinants apart from the antigen expression level that can also influence the ability of Ag+ cells to demonstrate the bystander effect in a co-culture system. The co-culture analysis also suggested that the bystander effect of the ADC can dissipate over the period of time as the population of Ag+ cells declines. A novel PD model was developed to mathematically characterize the bystander effect of ADCs by combining two different cell distribution models to represent the population of Ag+ and Ag− cells in a co-culture system. This PD model can be integrated with the systems PK model for ADCs in the future to generate a quantitative framework that is capable of supporting the discovery and development of novel ADCs with optimal bystander killing capabilities.












Similar content being viewed by others
References
Peters C, Brown S (2015) Antibody-drug conjugates as novel anti-cancer chemotherapeutics. Biosci Rep 35(4):e00225. doi:10.1042/BSR20150089
Sohayla RIQ, Robert S (2014) The clinical landscape of antibody-drug conjugates. J Antibody Drug Conjug. doi:10.14229/jadc.2014.8.1.001
Lin K, Tibbitts J (2012) Pharmacokinetic considerations for antibody drug conjugates. Pharm Res 29(9):2354–2366. doi:10.1007/s11095-012-0800-y
Chari RV, Miller ML, Widdison WC (2014) Antibody-drug conjugates: an emerging concept in cancer therapy. Angew Chem 53(15):3796–3827. doi:10.1002/anie.201307628
Younes A, Bartlett NL, Leonard JP, Kennedy DA, Lynch CM, Sievers EL, Forero-Torres A (2010) Brentuximab vedotin (SGN-35) for relapsed CD30-positive lymphomas. N Engl J Med 363(19):1812–1821. doi:10.1056/NEJMoa1002965
Girish S, Gupta M, Wang B, Lu D, Krop IE, Vogel CL, Burris Iii HA, LoRusso PM, Yi JH, Saad O, Tong B, Chu YW, Holden S, Joshi A (2012) Clinical pharmacology of trastuzumab emtansine (T-DM1): an antibody-drug conjugate in development for the treatment of HER2-positive cancer. Cancer Chemother Pharmacol 69(5):1229–1240. doi:10.1007/s00280-011-1817-3
Hamblett KJ, Senter PD, Chace DF, Sun MM, Lenox J, Cerveny CG, Kissler KM, Bernhardt SX, Kopcha AK, Zabinski RF, Meyer DL, Francisco JA (2004) Effects of drug loading on the antitumor activity of a monoclonal antibody drug conjugate. Clin Cancer Res 10(20):7063–7070. doi:10.1158/1078-0432.CCR-04-0789
Kamath AV, Iyer S (2015) Preclinical pharmacokinetic considerations for the development of antibody drug conjugates. Pharm Res 32(11):3470–3479. doi:10.1007/s11095-014-1584-z
Erickson HK, Park PU, Widdison WC, Kovtun YV, Garrett LM, Hoffman K, Lutz RJ, Goldmacher VS, Blattler WA (2006) Antibody-maytansinoid conjugates are activated in targeted cancer cells by lysosomal degradation and linker-dependent intracellular processing. Cancer Res 66(8):4426–4433. doi:10.1158/0008-5472.CAN-05-4489
Okeley NM, Miyamoto JB, Zhang X, Sanderson RJ, Benjamin DR, Sievers EL, Senter PD, Alley SC (2010) Intracellular activation of SGN-35, a potent anti-CD30 antibody-drug conjugate. Clin Cancer Res 16(3):888–897. doi:10.1158/1078-0432.CCR-09-2069
Katz J, Janik JE, Younes A (2011) Brentuximab Vedotin (SGN-35). Clin Cancer Res 17(20):6428–6436. doi:10.1158/1078-0432.CCR-11-0488
Kim EG, Kim KM (2015) Strategies and advancement in antibody-drug conjugate optimization for targeted cancer therapeutics. Biomol Ther 23(6):493–509. doi:10.4062/biomolther.2015.116
Fisher R, Pusztai L, Swanton C (2013) Cancer heterogeneity: implications for targeted therapeutics. Br J Cancer 108(3):479–485. doi:10.1038/bjc.2012.581
Seol H, Lee HJ, Choi Y, Lee HE, Kim YJ, Kim JH, Kang E, Kim SW, Park SY (2012) Intratumoral heterogeneity of HER2 gene amplification in breast cancer: its clinicopathological significance. Mod Pathol 25(7):938–948. doi:10.1038/modpathol.2012.36
Kovtun YV, Audette CA, Ye Y, Xie H, Ruberti MF, Phinney SJ, Leece BA, Chittenden T, Blattler WA, Goldmacher VS (2006) Antibody-drug conjugates designed to eradicate tumors with homogeneous and heterogeneous expression of the target antigen. Cancer Res 66(6):3214–3221. doi:10.1158/0008-5472.CAN-05-3973
Masuda S, Miyagawa S, Sougawa N, Sawa Y (2015) CD30-targeting immunoconjugates and bystander effects. Nat Rev Clin Oncol 12(4):245. doi:10.1038/nrclinonc.2014.159-c1
van der Lee MM, Groothuis PG, Ubink R, van der Vleuten MA, van Achterberg TA, Loosveld EM, Damming D, Jacobs DC, Rouwette M, Egging DF, van den Dobbelsteen D, Beusker PH, Goedings P, Verheijden GF, Lemmens JM, Timmers M, Dokter WH (2015) The preclinical profile of the duocarmycin-based HER2-targeting ADC SYD985 predicts for clinical benefit in low HER2-expressing breast cancers. Mol Cancer Ther 14(3):692–703. doi:10.1158/1535-7163.MCT-14-0881-T
Golfier S, Kopitz C, Kahnert A, Heisler I, Schatz CA, Stelte-Ludwig B, Mayer-Bartschmid A, Unterschemmann K, Bruder S, Linden L, Harrenga A, Hauff P, Scholle FD, Muller-Tiemann B, Kreft B, Ziegelbauer K (2014) Anetumab ravtansine: a novel mesothelin-targeting antibody-drug conjugate cures tumors with heterogeneous target expression favored by bystander effect. Mol Cancer Ther 13(6):1537–1548. doi:10.1158/1535-7163.MCT-13-0926
Li JY, Perry SR, Muniz-Medina V, Wang X, Wetzel LK, Rebelatto MC, Hinrichs MJ, Bezabeh BZ, Fleming RL, Dimasi N, Feng H, Toader D, Yuan AQ, Xu L, Lin J, Gao C, Wu H, Dixit R, Osbourn JK, Coats SR (2016) A biparatopic HER2-targeting antibody-drug conjugate induces tumor regression in primary models refractory to or ineligible for HER2-targeted therapy. Cancer Cell 29(1):117–129. doi:10.1016/j.ccell.2015.12.008
Lobo ED, Balthasar JP (2002) Pharmacodynamic modeling of chemotherapeutic effects: application of a transit compartment model to characterize methotrexate effects in vitro. AAPS Pharm Sci 4(4):E42. doi:10.1208/ps040442
Simeoni M, Magni P, Cammia C, De Nicolao G, Croci V, Pesenti E, Germani M, Poggesi I, Rocchetti M (2004) Predictive pharmacokinetic-pharmacodynamic modeling of tumor growth kinetics in xenograft models after administration of anticancer agents. Cancer Res 64(3):1094–1101
Yang J, Mager DE, Straubinger RM (2010) Comparison of two pharmacodynamic transduction models for the analysis of tumor therapeutic responses in model systems. AAPS J 12(1):1–10. doi:10.1208/s12248-009-9155-7
D’Argenio DZ, Schumitzky A, Wang X (2009) ADAPT 5 User’s Guide: Pharmacokinetic/Pharmacodynamic Systems Analysis Software. Biomedical Simulations Resource, Los Angeles
Tolcher AW, Ochoa L, Hammond LA, Patnaik A, Edwards T, Takimoto C, Smith L, de Bono J, Schwartz G, Mays T, Jonak ZL, Johnson R, DeWitte M, Martino H, Audette C, Maes K, Chari RV, Lambert JM, Rowinsky EK (2003) Cantuzumab mertansine, a maytansinoid immunoconjugate directed to the CanAg antigen: a phase I, pharmacokinetic, and biologic correlative study. J Clin Oncol 21(2):211–222
Ogitani Y, Hagihara K, Oitate M, Naito H, Agatsuma T (2016) Bystander killing effect of DS-8201a, a novel anti-HER2 antibody-drug conjugate, in tumors with HER2 heterogeneity. Cancer Sci. doi:10.1111/cas.12966
Maass KF, Kulkarni C, Betts AM, Wittrup KD (2016) Determination of cellular processing rates for a trastuzumab-maytansinoid antibody-drug conjugate (ADC) highlights key parameters for ADC design. AAPS J. doi:10.1208/s12248-016-9892-3
Perrino E, Steiner M, Krall N, Bernardes GJ, Pretto F, Casi G, Neri D (2014) Curative properties of noninternalizing antibody-drug conjugates based on maytansinoids. Cancer Res 74(9):2569–2578. doi:10.1158/0008-5472.CAN-13-2990
Singh AP, Maass KF, Betts AM, Wittrup KD, Kulkarni C, King LE, Khot A, Shah DK (2016) Evolution of antibody-drug conjugate tumor disposition model to predict preclinical tumor pharmacokinetics of trastuzumab-emtansine (T-DM1). AAPS J. doi:10.1208/s12248-016-9904-3
Shah DK, Haddish-Berhane N, Betts A (2012) Bench to bedside translation of antibody drug conjugates using a multiscale mechanistic PK/PD model: a case study with brentuximab-vedotin. J Pharmacokinet Pharmacodyn 39(6):643–659. doi:10.1007/s10928-012-9276-y
Acknowledgments
This work was supported by NIH Grant GM114179 to D.K.S., and the Centre for Protein Therapeutics at the State University of New York at Buffalo. S.S. was supported by a post-doctoral fellowship from Hoffman La-Roche, Switzerland to D.K.S. Authors would also like to thank Dr. Wojciech Krzyzanski, University at Buffalo, for his helpful discussion on mathematical modeling section of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Singh, A.P., Sharma, S. & Shah, D.K. Quantitative characterization of in vitro bystander effect of antibody-drug conjugates. J Pharmacokinet Pharmacodyn 43, 567–582 (2016). https://doi.org/10.1007/s10928-016-9495-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10928-016-9495-8