Abstract
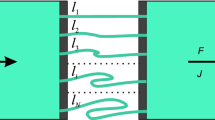
The utility of a circulatory three-compartment model for the assessment of tissue uptake kinetics is tested by comparison with the respective distributed models using pharmacokinetic data of rocuronium in patients These minimal physiologically based models have a common structure consisting of two subsystems representing the lung and the lumped systemic circulation, with two regions, the vascular and tissue space. The distributed models are based on either diffusion-limited tissue distribution, permeability-limited tissue uptake or the assumption of an empirical transit time density function. With a deviation in the estimate of the permeability-surface area product (PS) of about 18 %, the compartmental approach appears as a useful alternative on condition that a priori knowledge of cardiac output is included. It is also shown that the distribution clearance calculated from the parameters of a mammillary compartment model changes proportional to PS and can be used as an indirect measure of permeability-limited tissue uptake of drugs.





Similar content being viewed by others
Abbreviations
- C-RC:
-
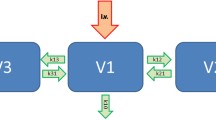
Minimal recirculatory compartmental model
- C-RCG:
-
Extended recirculatory compartmental model
- D-DL:
-
Distributed model: diffusision limited
- D-PL:
-
Distributed model: permeability limited
- D-TD:
-
Model based on empirical transit time density functions
- C-M3:
-
Mammillary three-compartment model
- ICG:
-
Indocyanine green
- AUC M :
-
Area under the curve in a hypothetical non-eliminating system
- C(t):
-
Concentration–time curve
- \(\hat{C}(s)\) :
-
Laplace transform of C(t)
- CL :
-
Elimination clearance
- CL M :
-
Distribution clearance
- d :
-
Diffusional equilibration time in tissue
- D iv :
-
Bolus intravenous dose
- \(\hat{f}_{i} (s)\) :
-
Laplace transform of transit time density (subsystem i)
- I(t):
-
Input rate
- MT i :
-
Mean transit time of subsystem i
- \(RD_{i}^{2}\) :
-
Squared relative transit time dispersion of subsystem i
- PS :
-
Permeability-surface area product
- Q :
-
Cardiac output
- V p ,V s :
-
Volumes of the pulmonary and systemic blood compartments
- V T,s :
-
Volume of the systemic tissue (V T,s) compartment
- V ss :
-
Steady-state distribution volume
- x i (t):
-
Drug amount in compartment i
References
Weiss M, Tura A, Kautzky-Willer A, Pacini G, D’Argenio DZ (2016) Human insulin dynamics in women: a physiologically based model. Am J Physiol Regul Integr Comp Physiol 310(3):R268–R274
Rowland M, Peck C, Tucker G (2011) Physiologically-based pharmacokinetics in drug development and regulatory science. Annu Rev Pharmacol Toxicol 51:45–73
Kuipers JA, Boer F, Olofsen E, Bovill JG, Burm AGL (2001) Recirculatory pharmacokinetics and pharmacodynamics of rocuronium in patients: the influence of cardiac output. Anesthesiology 94(1):47
Weiss M, Reekers M, Vuyk J, Boer F (2011) Circulatory model of vascular and interstitial distribution kinetics of rocuronium: a population analysis in patients. J Pharmacokinet Pharmacodyn 38(2):165–178
Weiss M, Krejcie TC, Avram MJ (2007) Circulatory transport and capillary-tissue exchange as determinants of the distribution kinetics of inulin and antipyrine in dog. J Pharm Sci 96(4):913–926
Sjöstrand M, Gudbjörnsdottir S, Holmäng A, Lönn L, Strindberg L, Lönnroth P (2002) Delayed transcapillary transport of insulin to muscle interstitial fluid in obese subjects. Diabetes 51(9):2742–2748
Weiss M, Roberts MS (1996) Tissue distribution kinetics as determinant of transit time dispersion of drugs in organs: application of a stochastic model to the rat hindlimb. J Pharmacokinet Pharmacodyn 24(2):173–196
Weiss M, Krejcie TC, Avram MJ (2006) Transit time dispersion in pulmonary and systemic circulation: effects of cardiac output and solute diffusivity. Am J Physiol Heart Circ Physiol 291(2):H861–H870
Weiss M, Pang KS (1992) Dynamics of drug distribution. I. Role of the second and third curve moments. J Pharmacokinet Pharmacodyn 20(3):253–278
D’Argenio DZSA, Wang X (2009) ADAPT 5 user’s guide: pharmacokinetic/pharmacodynamic systems analysis software. Biomedical Simulations Resource, Los Angeles
Schalla M, Weiss M (1999) Pharmacokinetic curve fitting using numerical inverse Laplace transformation. Eur J Pharm Sci 7(4):305–309
Weiss M, Krejcie TC, Avram MJ (2007) A minimal physiological model of thiopental distribution kinetics based on a multiple indicator approach. Drug Metab Dispos 35(9):1525–1532
Henthorn TK, Krejcie TC, Avram MJ (2008) Early drug distribution: a generally neglected aspect of pharmacokinetics of particular relevance to intravenously administered anesthetic agents. Clin Pharmacol Ther 84(1):18–22
Avram MJ, Krejcie TC (2003) Using front-end kinetics to optimize target-controlled drug infusions. Anesthesiology 99(5):1078–1086
Masui K, Upton RN, Doufas AG, Coetzee JF, Kazama T, Mortier EP, Struys MM (2010) The performance of compartmental and physiologically based recirculatory pharmacokinetic models for propofol: a comparison using bolus, continuous, and target-controlled infusion data. Anesth Analg 111(2):368–379
Upton R (2004) The two-compartment recirculatory pharmacokinetic model—an introduction to recirculatory pharmacokinetic concepts. Br J Anaesth 92(4):475–484
Weiss M (2007) Residence time dispersion as a general measure of drug distribution kinetics: estimation and physiological interpretation. Pharm Res 24(11):2025–2030
Weiss M (2008) How does obesity affect residence time dispersion and the shape of drug disposition curves? Thiopental as an example. J Pharmacokinet Pharmacodyn 35(3):325–336
Thompson MD, Beard DA (2011) Development of appropriate equations for physiologically based pharmacokinetic modeling of permeability-limited and flow-limited transport. J Pharmacokinet Pharmacodyn 38(4):405–421
Cao Y, Jusko WJ (2012) Applications of minimal physiologically-based pharmacokinetic models. J Pharmacokinet Pharmacodyn 39(6):711–723
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
When using models D-DL or D-PL model, first the arterial ICG concentration–time curve after rapid bolus injection (dose D iv ) is fitted to the following equation (given in the Laplace domain)
where \(\hat{f}_{B,p} (s)\) and \(\hat{f}_{B,s} (s)\) are the Laplace transforms of pulmonary and systemic TTDs (Eq. 6); Q and CL denote cardiac output and clearance, respectively. For fitting the C(t) data rocuronium also Eq. 10 is used, but \(\hat{f}_{B,s} (s)\) is replaced by f s (s), i.e., Equation 4 in case of the D-DL model, and by Eq. 5 for the D-PL model. The same holds for f B,p (s)(substituting V T,s and V B,s in Eqs. 4 and 5 by V T,p and V B,p , respectively. For the D-TD model, the f i (s)are given by Eq. 6.
In model D-PL (Eq. 5) the estimated relative dispersion in the systemic circulation and cardiac output determine CL M when the influence of the lungs is neglected [8] (Weiss, 2006)
Calculating AUC M defined by Eq. (8) from Eqs. 1–3 (for CL = 0), one obtains for the C-RC model C-RC:
For the C-M3 model CL M is given by [17]
Rights and permissions
About this article
Cite this article
Weiss, M. Comparison of distributed and compartmental models of drug disposition: assessment of tissue uptake kinetics. J Pharmacokinet Pharmacodyn 43, 505–512 (2016). https://doi.org/10.1007/s10928-016-9484-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10928-016-9484-y