Abstract
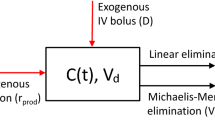
The current study aims to provide the closed form solutions of one-compartment open models exhibiting simultaneous linear and nonlinear Michaelis–Menten elimination kinetics for single- and multiple-dose intravenous bolus administrations. It can be shown that the elimination half-time (\(t_{1/2}\)) has a dose-dependent property and is upper-bounded by \(t_{1/2}\) of the first-order elimination model. We further analytically distinguish the dominant role of different elimination pathways in terms of model parameters. Moreover, for the case of multiple-dose intravenous bolus administration, the existence and local stability of the periodic solution at steady state are established. The closed form solutions of the models are obtained through a newly introduced function motivated by the Lambert W function.








Similar content being viewed by others
References
Craig M, Humphries AR, Nekka F, Bélair J, Li J Mackey MC (2014) Neutrophil dynamics during concurrent chemotherapy and G-CSF administration: mathematical modelling guides dose optimisation to minimize neutropenia. J Theor Biol (to appear)
Foley C, Mackey MC (2009) Mathematical model for G-CSF administration after chemotherapy. J Theor Biol 257:27–44
Krzyzanski W, Wiczling P, Lowe P, Pigeolet E, Fink M, Berghout A, Balser S (2010) Population modeling of filgrastim PK-PD in healthy adults following intravenous and subcutaneous administrations. J Clin Pharmacol 50:101S–112S
Scholz M, Schirm S, Wetzler M, Engel C, Loeffler M (2012) Pharmacokinetic and -dynamic modelling of G-CSF derivatives in humans. Theor Biol Med Model 9:32
Kozawa S, Yukawa N, Liu J, Shimamoto A, Kakizaki E, Fujimiya T (2007) Effect of chronic ethanol administration on disposition of ethanol and its metabolites in rat. Alcohol 41(2):87–93
Woo S, Krzyzanski W, Jusko WJ (2006) Pharmacokinetic and pharmacodynamic modeling of recombinant human erythropoietin after intravenous and subcutaneous administration in rats. J Pharmacol Exp Ther 319(3):1297–306
Tang S, Xiao Y (2007) One-compartment model with Michaelis-Menten elimination kinetics and therapeutic window: an analytical approach. J Pharmacokinet Pharmacodyn 34:807–827
Cheng HY, Jusko WJ (1989) Mean residence time of drugs showing simultaneous first-Order and Michaelis-Menten elimination kinetics. Pharm Res 6(3):258–61
Wagner JG, Szpunar GJ, Ferry JJ (1985) Michaelis-Menten elimination kinetics: areas under curves, steady-state concentrations, and clearances for compartment models with different types of input. Biopharm Drug Dispos 6(2):177–200
Ritschel WA, Kearns GL (2004) Handbook of basic pharmacokinetics..including clinical applications, 6th edn. American Pharmacists Association, Washington
Beal SL (1982) On the solution to the Michaelis-Menten equation. J Pharmacokin Biopharm 10:109–119
Beal SL (1983) Computation of the explicit solution to the Michaelis-Menten equation. J Pharmacokinet Biopharm 11:641–657
Sonnad JR, Goudar CT (2009) Solution of the Michaelis-Menten equation using the decomposition method. Math Biosci Eng 6(1):173–188
Schnell S, Mendoza C (1997) Closed form solution for time-dependent enzyme kinetics. J Theor Biol 187:207–212
Corless RM, Gonnet GH, Hare DEG, Jeffrey DJ, Knuth DE (1996) On the Lambert W function. Adv Comput Math 5:329–359
Hallifax D, Rawden HC, Hakooz N, Houston JB (2005) Prediction of metabolic clearance using cryopreserved human hepatocytes: kinetic characteristics for five benzodiazepines. Drug Metab Dispos 33(12):1852–1858
Gibaldi M, Perrier D (2007) Pharmacokinetics, 2nd edn. Informa Healthcare, New York
Wang YM, Sloey B, Wong T, Khandelwal P, Melara R, Sun YN (2011) Investigation of the pharmacokinetics of romiplostim in rodents with a focus on the clearance mechanism. Pharm Res 28:1931–1938
Kotto-Kome AC, Fox SE, Lu W, Yang BB, Christensen RD, Calhoun DA (2004) Evidence that the granulocyte colony-stimulating factor (G-CSF) receptor plays a role in the pharmacokinetics of G-CSF and PegG-CSF using a G-CSF-R KO model. Pharmacol Res 50:55–58
Acknowledgments
The authors thank the referees for their careful reading of the manuscript and valuable comments they made that helped us to improve the paper. The authors acknowledge the financial support of NSERC-Industrial Chair in Pharmacometrics, FRQNT, NSERC, Mprime, Novartis, Pfizer and InVentiv Health.
Author information
Authors and Affiliations
Corresponding author
Appendices
Appendix A
Definition 2
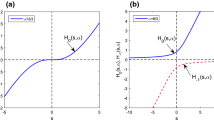
For given parameters \(v\in (0,1)\), \(a\in (0,\infty )\) and \(b\in (0,\infty )\), let us define \(Y(t,v,a,b)\) as the inverse function of \(\displaystyle h(s)=\left( \frac{s}{s+a}\right) ^{1-v}\left( \frac{s+b}{s+a+b}\right) ^v\), for \(s>0\), which means
For any given \(v\in (0,1)\), \(a\in (0,\infty )\) and \(b\in (0,\infty )\), the derivative of \(h(s)\) is
Thus \(h(s)\) is a continuous, differentiable and one-to-one map with respect to the variable \(s>0\) such that the inverse function of \(h(s)\) exists and is globally unique. Moreover, we can verify that \(h(0)=0\) and \(\lim \limits _{s\rightarrow \infty }h(s)=1\).
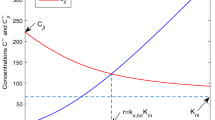
In the following, we show how we use \(h(s)\) and \(e^{-k_{el}\tau }\) to ensure the existence and uniqueness of \(C^*\). Here for each dosing interval \(\tau \) we have \(e^{-k_{el}\tau }\in (0,1)\) such that there exists a unique \(C^*\) satisfying Eq. 29. Moreover, \(C^*\) is a monotonically decreasing function with respect to dosing interval \(\tau \). In other words, the longer is the dosing interval, the smaller is the value of \(e^{-k_{el}\tau }\) and then the smaller is the solution \(C^*\) to be obtained (Fig. 9). However, if the dosing interval \(\tau \) is fixed, \(C^*\) would be monotonically increasing with the dose \(D\) (Fig. 9).
Unique existence of the solution \(C^*\) of Eq. 29: \(C^*\) is an increasing function with respect to dose \(D\), here we take \(CL_l= 0.4\) ml/h, \(K_m = 1\) \(\upmu \)g/ml, \(V_{max} = 0.4\) \(\upmu \)g/h, \(V_d = 1\,\,ml\); \(\tau = 1\) h; \(D_{small} = 1\) \(\upmu \)g and \(D_{large}=3\,\,ug\)
Appendix B
In the following, we show that the periodic solution given by Eqs. 31 is locally asymptotically stable.
Theorem 1
The periodic solution given by Eqs. 31 is locally stable.
Proof
The model solutions described by Eqs. 20–21 will asymptotically approach the periodic solution given by Eqs. 31, for any given initial dose.
In terms of Eq. 25, we define a stroboscopic map \({\mathcal {M}}: C((n-1)\tau ^+)\in (0,\infty )\longmapsto C(n\tau ^+)\in (0,\infty )\) in the sense that
We have shown that the fixed point of the map \({\mathcal {M}}(C((n-1)\tau ^+))\), denoted \(C^{**}=C^*+D/V_d\) and \(C^*\) expressed by Eq. 30, uniquely exists. Then solution \(C^{**}\) at equilibrium is locally stable provided that the following condition
is satisfied.
To show this, let \(\displaystyle z=g(C((n-1)\tau ^+),n\tau )\), and according to the chain rule, we have
On the one hand, replacing \(t\) by \(n\tau \) in the definition of \(g\) function, we obtain
On the other hand, it follows from Eq. 25 that we have
Moreover, the definition of \(X\) function yields
Substituting Eqs. 45 and 46 into Eq. 11 yields
Substituting Eqs. 44, 47 into Eq. 43, we have
which implies the local stability of the steady-state \(C^{**}\). \(\square \)
Rights and permissions
About this article
Cite this article
Wu, X., Li, J. & Nekka, F. Closed form solutions and dominant elimination pathways of simultaneous first-order and Michaelis–Menten kinetics. J Pharmacokinet Pharmacodyn 42, 151–161 (2015). https://doi.org/10.1007/s10928-015-9407-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10928-015-9407-3