Abstract
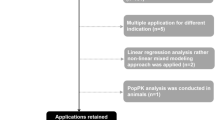
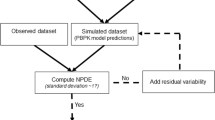
Population pharmacokinetic (popPK) analyses are at the core of Pharmacometrics and need to be performed regularly. Although these analyses are relatively standard, a large variability can be observed in both the time (efficiency) and the way they are performed (quality). Main reasons for this variability include the level of experience of a modeler, personal preferences and tools. This paper aims to examine how the process of popPK model building can be supported in order to increase its efficiency and quality. The presented approach to the conduct of popPK analyses is centered around three key components: (1) identification of most common and important popPK model features, (2) required information content and formatting of the data for modeling, and (3) methodology, workflow and workflow supporting tools. This approach has been used in several popPK modeling projects and a documented example is provided in the supplementary material. Efficiency of model building is improved by avoiding repetitive coding and other labor-intensive tasks and by putting the emphasis on a fit-for-purpose model. Quality is improved by ensuring that the workflow and tools are in alignment with a popPK modeling guidance which is established within an organization. The main conclusion of this paper is that workflow based approaches to popPK modeling are feasible and have significant potential to ameliorate its various aspects. However, the implementation of such an approach in a pharmacometric organization requires openness towards innovation and change—the key ingredient for evolution of integrative and quantitative drug development in the pharmaceutical industry.




Similar content being viewed by others
References
Bonate PL (2011) Pharmacokinetic-pharmacodynamic modeling and simulation, 2nd edn. Springer, New York
Byon W, Smith MK, Chan P, Tortorici MA, Riley S, Dai H, Dong J, Ruiz-Garcia A, Sweeney K, Cronenberger C (2013) Establishing best practices and guidance in population modeling: an experience with an internal population pharmacokinetic analysis guidance. CPT 2:e51
Ette EI, Williams PJ (2004) Population pharmacokinetics I: background, concepts, and models. Ann Pharmacother 8(10):1702–1706
Mould DR, Upton RN (2013) Basic concepts in population modeling, simulation, and model-based drug development-part 2: introduction to pharmacokinetic modeling methods. CPT 2:e38
Vozeh S, Steimer J-L, Rowland M, Morselli P, Mentre F, Balant LP, Aarons L (1996) The use of population pharmacokinetics in drug development. Clin Pharmacokinet 30(2):81–93
Samara E, Granneman R (1997) Role of population pharmacokinetics in drug development. A pharmaceutical industry perspective. Clin Pharmacokinet 32:294–312
Sun H, Fadiran EO, Jones CD, Lesko L, Huang SM, Higgins K, Hu C, Machado S, Maldonado S, Williams R, Hossain M, Ette EI (1999) Population pharmacokinetics. A regulatory perspective. Clin Pharmacokinet 37(1):41–58
Gobburu JVS (2010) Pharmacometrics 2020. J Clin Pharmacol 50:151S–157S
Romero K, Corrigan B, Tornoe C, Gobburu JV, Danhof M, Gillespie WR, Gastonguay MR, Meibohm B, Derendorf H (2010) Pharmacometrics as a discipline is entering the “industrialization” phase: standards, automation, knowledge sharing, and training are critical for future success. J Clin Pharmacol 509:9S–19S
Jönsson S, Jonsson N (2007) Timing and efficiency in population pharmacokinetic/pharmacodynamic data analysis projects. In: Pharmacometrics: the science of quantitative pharmacology, Chapter 11. Wiley Inc, New Jersey
U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research, Center for Biologics Evaluation and Research (1999) Guidance for industry population pharmacokinetics. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm072137.pdf. Accessed 4 February 2014
European Medicines Agency and Committee for Medicinal Products for Human Use (2007) Guideline on the reporting the results of population pharmacokinetic analyses. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003067.pdf. Accessed 4 February 2014
Bonate PL, Strougo A, Desai A, Roy M, Yassen A, van der Walt JS, Kaibara A, Tannenbaum S (2012) Guidelines for the quality control of population pharmacokinetic-pharmacodynamic analyses: an industry perspective. AAPS J 14:749–758
Beal SL, Sheiner LB (1992) NONMEM User’s Guides. NONMEM Project Group University of California, San Francisco
Kiang TK, Sherwin CM, Spigarelli MG, Ensom MH (2012) Fundamentals of population pharmacokinetic modelling: modelling and software. Clin Pharmacokinet 51:515–525
Bergsma TT, Knebel W, Fisher J, Gillespie WR, Riggs MM, Gibiansky L, Gastonguay MR (2013) Facilitating pharmacometric workflow with metrumrg package for R. Comput Methods Programs Biomed 109:77–85
R development core team (2008) R: a language and environment for statistical computing. R Foundationn for Statistical Computing, Vienna. ISBN 3-900051-07-0
Ridolfi L, Franklin C, Della Pasqua O (2013) Predictive modelling environment—infrastructure and functionality for pharmacometric activities in R&D. PAGE meeting, Glasgow [http://www.page-meeting.org/default.asp?abstract=2935]
Schaap J, Verhoeven S, Vogel G, Rooseboom M, van Schaik R (2007) Automation of structural pharmacokinetic model search in NONMEM: evaluation with preclinical datasets. PAGE meeting, Copenhagen [www.page-meeting.org/?abstract=1188]
MATLAB, Mathworks [http://www.mathworks.com]
Schmidt H (2013) SBPOP Package: efficient support for model based drug development—from mechanistic models to complex trial simulation. PAGE meeting, Glasgow [http://www.page-meeting.eu/default.asp?abstract=2670], [http://www.sbtoolbox2.org]
Monolix, Lixoft [http://www.lixoft.eu]
Gastonguay MR (2004) A full model estimation approach for covariate effects: inference based on clinical importance and estimation precision. AAPS J 6(S1):W4354
Acknowledgments
The authors acknowledge Novartis colleagues from the Advanced Quantitative Sciences (AQS) group, who provided valuable input, discussion, and feedback. Special thanks go to Anne-Gaëlle Dosne and Ivan Demin who reviewed the paper in detail and helped to make it much better.
Conflict of interest
Henning Schmidt and Andrijana Radivojevic are employees of Novartis Pharma AG and receive salaries and benefits commensurate with employment.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Schmidt, H., Radivojevic, A. Enhancing population pharmacokinetic modeling efficiency and quality using an integrated workflow. J Pharmacokinet Pharmacodyn 41, 319–334 (2014). https://doi.org/10.1007/s10928-014-9370-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10928-014-9370-4