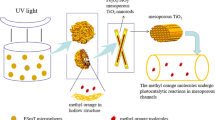
Abstract
Novel Fe3O4@SiO2@mTiO2–Au (mTiO2: mesoporous TiO2) core–shell microspheres were fabricated by the facile and efficient methods, and they were characterized by SEM, TEM, XRD, XPS, BET, VSM, and photoelectrochemical measurements. Through the test of methylene blue (MB) degradation, the relative photocatalytic activity of four samples follows the order: Fe3O4@SiO2@mTiO2–Au (FSTA) > Fe3O4@SiO2@mTiO2 (FST) > Fe3O4@mTiO2 (FT) > P25. The superior photocatalytic property of FSTA is mainly attributed to the positive effect of three components including decorative Au nanoparticles, SiO2 intermediate layer, and mesoporous TiO2 layer. Moreover, FSTA microspheres were easily recycled by using an external magnetic field while their photocatalytic efficiency had no obvious decrease after running 6 times of photocatalytic test. The well-designed microstructure makes FSTA a highly efficient, recoverable, and stable photocatalytic system, which has promising applications in environmental treatment.











Similar content being viewed by others
References
Mohan D, Pittman CU (2007) Arsenic removal from water/wastewater using adsorbents–a critical review. J Hazard Mater 142:1–53
Xu XY, Zhou XS, Zhang LL et al (2015) Photoredox degradation of different water pollutants [MO, RhB, MB, and Cr(VI)] using Fe–N–S–tri-doped TiO2 nanophotocatalyst prepared by novel chemical method. Mater Res Bull 70:106–113
Zhu F, Li CP, Ha MN et al (2016) Molten-salt synthesis of Cu–SrTiO3/TiO2 nanotube heterostructures for photocatalytic water splitting. J Mater Sci 51:4639–4649
Dong XL, Shao Y, Zhang XX, Ma HC, Zhang XF, Shi F, Ma C, Xue M (2013) Synthesis and properties of magnetically separable Fe3O4/TiO2/Bi2O3 photocatalysts. Res Chem Intermed 40:2953–2961
Hong K, Yoo KS (2011) Synthesis of TiO2 hollow spheres using binary ionic liquids as an electrocatalyst. Res Chem Intermed 37:1325–1331
Shen JY, Wu YN, Fu L et al (2014) Preparation of doped TiO2 nanofiber membranes through electrospinning and their application for photocatalytic degradation of malachite green. J Mater Sci 49:2303–2314
Habibi MH, Nasr-Esfahani M, Egerton TA, Anpo M (2007) Preparation, characterization and photocatalytic activity of TiO2/methylcellulose nanocomposite films derived from nanopowder TiO2 and modified sol–gel titania. J Mater Sci 42:6027–6035
Almeida NA, Martins PM, Teixeira S et al (2016) TiO2/graphene oxide immobilized in P(VDF-TrFE) electrospun membranes with enhanced visible-light-induced photocatalytic performance. J Mater Sci 51:6974–6986
Xiang QJ, Lv K, Yu JG (2010) Pivotal role of fluorine in enhanced photocatalytic activity of anatase TiO2 nanosheets with dominant (001) facets for the photocatalytic degradation of acetone in air. Appl Catal B 96:557–564
Enesca A, Baneto M, Perniu D et al (2016) Solar-activated tandem thin films based on CuInS2, TiO2 and SnO2 inoptimized wastewater treatment processes. Appl Catal B 186:69–76
Chen XB, Mao SS (2007) Titanium dioxide nanomaterials: synthesis, properties, modifications, and applications. Chem Rev 107:2891–2959
Gong XQ, Selloni A (2005) Reactivity of anatase TiO2 nanoparticles: the role of the minority (001) surface. J Phys Chem B 109:19560–19562
Yu XX, Yu JG, Cheng B, Jaroniec M (2009) Synthesis of hierarchical flower-like AlOOH and TiO2/AlOOH superstructures and their enhanced photocatalytic properties. J Phys Chem C 113:17527–17535
Naraginti S, Thejaswini TV, Prabhakaran D et al (2015) Enhanced photo-catalytic activity of Sr and Ag co-doped TiO2 nanoparticles for the degradation of direct green-6 and reactive blue-160 under UV and visible light. Spectrochim Acta A 149:571–579
Subramanian V, Wolf EE, Kamat PV (2004) Catalysis with TiO2@gold nanocomposites. Effect of metal particle size on the fermi level equilibration. J Am Chem Soc 126:4943–4950
Harifi T, Montazer M (2015) A robust super-paramagnetic TiO2:Fe3O4: Ag nanocomposite with enhanced photo and bio activities on polyester fabric via one step sonosynthesis. Ultrason Sonchem 27:543–551
Deng H, Li XL, Peng Q et al (2005) Monodisperse magnetic single-crystal ferrite microspheres. Angew Chem Int Ed 44:2782–2785
Deng YH, Deng CH, Qi DW et al (2009) Synthesis of core/shell colloidal magnetic zeolite microspheres for the immobilization of trypsin. Adv Mater 21:1377–1382
Deng YH, Qi DW, Deng CH, Zhang XM, Zhao DY (2008) Superparamagnetic high-magnetization microspheres with an Fe3O4@SiO2 core and perpendicularly aligned mesoporous SiO2 shell for removal of microcystins. J Am Chem Soc 130:28–29
Yu XX, Liu SW, Yu JG (2011) Superparamagnetic γ–Fe2O3@SiO2@TiO2 composite microspheres with superior photocatalytic properties. Appl Catal B 104:12–20
Dou YP, Liu HL, Peng JJ et al (2016) A green method for preparation of CNT/CS/AgNP composites and evaluation of their catalytic performance. J Mater Sci 51:5685–5694
Qin YY, Li YL, Tian Z et al (2016) Efficiently visible-light driven photoelectrocatalytic oxidation of As (III) at low positive biasing using Pt/TiO2 nanotube electrode. Nanoscale Res Lett 11:32–45
Pandikumar A, Murugesan S, Ramaraj R (2010) Functionalized silicate sol-gel-supported TiO2-Au core-shell nanomaterials and their photoelectrocatalytic activity. ACS Appl Mater Interfaces 2:1912–1917
Chi Y, Yuan Q, Li YJ et al (2013) Magnetically separable Fe3O4@SiO2@TiO2–Ag microspheres with well-designed nanostructure and enhanced photocatalytic activity. J Hazard Mater 262:404–411
Li X, Liu D, Song S, Zhang H (2014) Fe3O4@SiO2@TiO2@Pt hierarchical core-shell microspheres: controlled synthesis, enhanced degradation system, and rapid magnetic separation to recycle. Cryst Growth Des 14:5506–5511
Ma WF, Zhang Y, Li LL et al (2012) Tailor-made magnetic Fe3O4@mTiO2 microspheres with a tunable mesoporous anatase shell for highly selective and effective enrichment of phosphopeptides. ACS Nano 6:3179–3188
Zhu SY, Liang SJ, Gu Q et al (2012) Effect of Au supported TiO2 with dominant exposed 001 facets on the visible-light photocatalytic activity. Appl Catal B 119–120:146–155
Kruse N, Chenakin S (2011) XPS characterization of Au/TiO2 catalysts: binding energy assessment and irradiation effects. Appl Catal A 391:367–376
Okazaki K, Ichikawa S, Maeda Y, Haruta M, Kohyama M (2005) Electronic structures of Au supported on TiO2. Appl Catal A 291:45–54
Vijay A, Mills G, Metiu H (2003) Adsorption of gold on stoichiometric and reduced rutile TiO2 (110) surfaces. J Chem Phys 118:6536–6551
Kruk M, Jaroniec M (2001) Gas adsorption characterization of ordered organic-inorganic nanocomposite materials. Chem Mater 13:3169–3183
Yu JG, Yu JC, Leung MK et al (2003) Effects of acidic and basic hydrolysis catalysts on the photocatalytic activity and microstructures of bimodal mesoporous titania. J Catal 217:69–78
Yu JG, Yu JC, Ho WK et al (2003) Effects of alcohol content and calcination temperature on the textural properties of bimodally mesoporous titania. Appl Catal A 255:309–320
Liu SW, Yu JG, Jaroniec M (2010) Tunable photocatalytic selectivity of hollow TiO2 microspheres composed of anatase polyhedra with exposed 001 facets. J Am Chem Soc 132:11914–11916
Yu JG, Wang B (2010) Effect of calcination temperature on morphology and photoelectrochemical properties of anodized titanium dioxide nanotube arrays. Appl Catal B 94:295–302
Yu JG, Yu HG, Cheng B et al (2003) The effect of calcination temperature on the surface microstructure and photocatalytic activity of TiO2 thin films prepared by liquid phase deposition. J Phys Chem B 107:13871–13879
Zhou MH, Yu JG, Liu SW et al (2009) Spray-hydrolytic synthesis of highly photoactive mesoporous anatase nanospheres for the photocatalytic degradation of toluene in air. Appl Catal B 89:160–166
Liao G, Chen S, Quan X, Zhang Y, Zhao H (2011) Remarkable improvement of visible light photocatalysis with PANI modified core-shell mesoporous TiO2 microspheres. Appl Catal B 102:126–131
Yang WG, Wan FR, Chen QW, Li JJ, Xu DS (2010) Controlling synthesis of well-crystallized mesoporous TiO2 microspheres with ultrahigh surface area for high-performance dye-sensitized solar cells. J Mater Chem 20:2870–2876
Hagfeldt A, Gräetzel M (1995) Light-induced redox reactions in nanocrystalline systems. Chem Rev 95:49–68
Kamat PV (2008) Quantum dot solar cells. Semiconductor nanocrystals as light harvesters. J Phys Chem C 112:18737–18753
Mwaura JK, Zhao XY, Jiang H, Schanze KS, Reynolds JR (2006) Spectral broadening in nanocrystalline TiO2 solar cells based on poly(p-phenylene ethynylene) and polythiophene sensitizers. Chem Mater 18:6109–6111
Beydoun D, Ama R, Low GKC, Mcevoy S (2000) Novel photocatalyst: titania-coated magnetite. Activity and photodissolution. J Phys Chem B 104:4387–4396
Pearson A, Bhargava SK, Bansal V (2011) UV-switchable polyoxometalate sandwiched between TiO2 and metal nanoparticles for enhanced visible and solar light photococatalysis. J Am Chem Soc 27:9245–9252
Acknowledgments
This work is supported by the National Programs for High Technology Research and Development of China (863) (Item No. 2013AA032202), National Natural Science Foundation of China (Grant Nos. 61378085, 11404137 and 61308095), Program for the development of Science and Technology of Jilin province (Item No. 20130102004JC, and 20140101205JC), Program for the development of Science and Technology of Siping City ((Item No. 2015065).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, D., Yang, J., Li, X. et al. Preparation of magnetic Fe3O4@SiO2@mTiO2–Au spheres with well-designed microstructure and superior photocatalytic activity. J Mater Sci 51, 9602–9612 (2016). https://doi.org/10.1007/s10853-016-0167-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-016-0167-2