Abstract
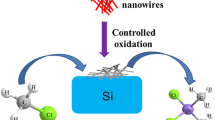
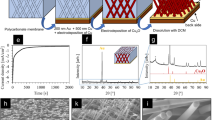
Cu2O nanowire mesocrystals (NWMCs), which possess well-defined octahedral morphology and high-porosity architecture with anisotropic interpenetrating nanowires, have attracted considerable attention owing to their superior physical and chemical properties. However, the current synthetic approach for Cu2O NWMCs using graphene oxide as modifier leads to uncontrollable products and low productivity (~30 %), which largely hinder their further industrial application. Herein, we report a modified synthetic approach for controllable and large-scale preparation of Cu2O NWMCs using perylene-3,4,9,10-tetracarboxylic dianhydride molecule as modifier. The effects of growth time and initial pH on the morphology of final products have been systemically investigated. Under the optimal reaction condition with initial pH of 5.3 and reaction time of 15 h, the well-defined octahedral Cu2O NWMCs can be obtained with a productivity as high as ~75 %. In addition, this synthetic approach can be easily scaled up from 50 to 500 mL autoclave. The ability of high productivity and reproducibility in synthesis of Cu2O NWMCs enable us to further study their peroxidase-like activity by catalyzing the oxidation of o-phenylenediamine in the presence of H2O2. The Cu2O NWMCs have exhibited a superior catalytic activity with the K cat of 1.14 × 10−2, 10 times higher than that of horseradish peroxidase. Moreover, the Cu2O NWMCs can also retain 69.5 % of their initial activities after 10-batch redox reactions. Our results provide a facile approach to controllable and large-scale synthesis of Cu2O NWMCs using homogeneous molecule modifier and open up opportunities to use Cu2O NWMCs as nanozyme for future industrial application.






Similar content being viewed by others
References
Cölfen H, Antonietti M (2005) Mesocrystals: inorganic superstructures made by highly parallel crystallization and controlled alignment. Angew Chem Int Ed 44:5576–5591
Song RQ, Cölfen H (2010) Mesocrystals-ordered nanoparticle superstructures. Adv Mater 22:1301–1330
Fang JX, Ding BJ, Gleiter H (2011) Mesocrystals: syntheses in metals and applications. Chem Soc Rev 40:5347–5360
Cöelfen H, Antonietti M (2008) Mesocrystals and nonclassical crystallization. Wiley, Chichester
Liu YQ, Zhang Y, Wang J (2014) Mesocrystals as a class of multifunctional materials. CrystEngComm 16:5948–5967
Ma MG, Cölfen H (2014) Mesocrystals-applications and potential. Curr Opin Colloid Interface Sci 19:56–65
Tachikawa T, Majima T (2014) Metal oxide mesocrystals with tailored structures and properties for energy conversion and storage applications. NPG Asia Mater 6:e100
Xu AW, Antonietti M, Cölfen H, Fang YP (2006) Uniform hexagonal plates of vaterite CaCO3 mesocrystals formed by biomimetic mineralization. Adv Funct Mater 16:903–908
Zhu GX, Liu YJ, Ji ZY, Bai S, Shen XP, Xu Z (2012) Hierarchical ZnO microspheres built by sheet-like network: large-scale synthesis and structurally enhanced catalytic performances. Mater Chem Phys 132:1065–1070
Gu CD, Zheng H, Wang XL, Tu JP (2015) Superior ethanol-sensing behavior based on SnO2 mesocrystals incorporating orthorhombic and tetragonal phases. RSC Adv 5:9143–9153
Uchiyama H, Hosono E, Honma I, Zhou HS, Imai H (2008) A nanoscale meshed electrode of single-crystalline SnO for lithium-ion rechargeable batteries. Electrochem Commun 10:52–55
Dai H, Zhang SP, Hong ZS, Li XH, Xu GF, Lin YY, Chen GN (2014) Enhanced photoelectrochemical activity of a hierarchical-ordered TiO2 mesocrystal and its sensing application on a carbon nanohorn support scaffold. Anal Chem 86:6418–6424
Wu XY, Yin S, Liu B, Kobayashi M, Kakihana M, Sato T (2014) A carbon modified NaTaO3 mesocrystal nanoparticle with excellent efficiency of visible light induced photocatalysis. J Mater Chem A 2:20832–20840
Yang JC, He Q, Zhu YM, Lin JC, Liu HJ, Hsieh YH, Wu PC, Chen YL, Lee SF, Chin YY, Lin HJ, Chen CT, Zhan Q, Arenholz E, Chu YH (2014) Magnetic mesocrystal-assisted magneto resistance in manganite. Nano Lett 14:6073–6079
Duan XC, Xiao SH, Wang LL, Huang H, Liu Y, Li QH, Wang TH (2015) Ionic liquid-modulated preparation of hexagonal tungsten trioxide mesocrystals for lithium-ion batteries. Nanoscale 7:2230–2234
Ihli J, Bots P, Kulak A, Benning LG, Meldrum FC (2013) Elucidating mechanisms of diffusion-based calcium carbonate synthesis leads to controlled mesocrystal formation. Adv Funct Mater 23:1965–1973
Liu L, Jiang J, Yu SH (2014) Polymorph selection and structure evolution of CaCO3 mesocrystals under control of poly (sodium 4-styrenesulfonate): synergetic effect of temperature and mixed solvent. Cryst Growth Des 14:6048–6056
Yang SH, Song XF, Zhang P, Sun J, Gao L (2014) Self-assembled α-Fe2O3 mesocrystals/graphene nanohybrid for enhanced electrochemical capacitors. Small 10:2270–2279
Barik R, Tripathy SK, Mohapatra M (2014) Hierarchical pseudo-cubic hematite nanoparticle as formaldehyde sensor. J Mater Sci 49:5345–5354. doi:10.1007/s10853-014-8237-9
Ye JF, Liu W, Cai JG, Chen S, Zhao XW, Zhou HH, Qi LM (2010) Nanoporous anatase TiO2 mesocrystals: additive-free synthesis, remarkable crystalline-phase stability, and improved lithium insertion behavior. J Am Chem Soc 133:933–940
Jin RC, Zhou JH, Guan YS, Liu H, Chen G (2014) Mesocrystal Co9S8 hollow sphere anodes for high performance lithium ion batteries. J Mater Chem A 2:13241–13244
Deng SZ, Tjoa V, Fan HM, Tan HR, Sayle DC, Olivo M, Mhaisalkar S, Wei J, Sow CH (2012) Reduced graphene oxide conjugated Cu2O nanowire mesocrystals for high performance NO2 gas sensor. J Am Chem Soc 134:4905–4917
Yue GH, Zhang Y, Zhang XQ, Wang CG, Zhao YC, Peng DL (2015) Synthesis of Cu2O mesocrystal and its application in photocatalysis. Appl Phys A 118:763–767
Deng SZ, Cherian CT, Liu XL, Tan HR, Yeo LH, Yu XJ, Rusydi A, Chowdari BVR, Fan HM, Sow CH (2014) Ultrathin hexagonal hybrid nanosheets synthesized by graphene oxide-assisted exfoliation of β-Co(OH)2 mesocrystals. Chem Eur J 20:12444–12452
Su DW, Dou SX, Wang GX (2014) Mesocrystal Co3O4 nanoplatelets as high capacity anode materials for Li-ion batteries. Nano Res 7:794–803
Liu YR, Bai J, Ma XJ, Li JF, Xiong SL (2014) Formation of quasi-mesocrystal ZnMn2O4 twin microspheres via an oriented attachment for lithium-ion batteries. J Mater Chem A 2:14236–14244
Dreyer DR, Todd AD, Bielawski CW (2014) Harnessing the chemistry of graphene oxide. Chem Soc Rev 43:5288–5301
Swarbrick JC, Rogers BL, Champness NR, Beton PH (2006) Hydrogen-bonded PTCDA-melamine networks and mixed phases. J Phys Chem B 110:6110–6114
Tautz FS (2007) Structure and bonding of large aromatic molecules on noble metal surfaces: the example of PTCDA. Prog Surf Sci 82:479–520
Tan YW, Xue XY, Peng Q, Zhao H, Wang TH, Li YD (2007) Controllable fabrication and electrical performance of single crystalline Cu2O nanowires with high aspect ratios. Nano Lett 7:3723–3728
Pandey M, Joshi GM, Deshmukh K, Ghosh NN, Raj NAN (2015) Electrical conductivity, optical properties and mechanical stability of 3, 4, 9, 10-perylenetetracarboxylic dianhidride based organic semiconductor. J Phys Chem Solids 80:52–61
Seto K, Pham J, Furukawa Y (2012) Infrared study on the molecular orientation in bulk-heterojunction films based on perylene and 3, 4, 9, 10-perylenetetracarboxylic dianhydride. Chem Phys Lett 529:31–34
Yang LH, Lv J, Sui YM, Fu WY, Zhou XM, Ma JW, Su S, Zhang WJ, Lv P, Wu D, Mu YN, Yang HB (2014) Fabrication of Cu2O/Ag composite nanoframes as surface-enhanced Raman scattering substrates in a successive one-pot procedure. CrystEngComm 16:2298–2304
Wang F, Wang GC, Yang S, Li CZ (2008) Layer-by-layer assembly of aqueous dispersible, highly conductive poly(aniline-co-o-anisidine)/poly(sodium 4-styrenesulfonate)/MWNTs core–shell nanocomposites. Langmuir 24:5825–5831
Mallick K, Witcomb M, Scurrell M (2006) Fabrication of a nanostructured gold-polymer composite material. Eur Phys J E 20:347–353
Chanda K, Rej S, Huang MH (2013) Investigation of facet effects on the catalytic activity of Cu2O nanocrystals for efficient regioselective synthesis of 3,5-disubstituted isoxazoles. Nanoscale 5:12494–12501
Gao ZY, Liu JL, Chang JL, Wu DP, He JJ, Wang K, Xu F, Jiang K (2012) Mesocrystalline Cu2O hollow nanocubes: synthesis and application in non-enzymatic amperometric detection of hydrogen peroxide and glucose. CrystEngComm 14:6639–6646
Schmidt A, Chau LK, Valencia VS, Armstrong NR (1995) Periodic multilayers of perylene-3,4,9,10-tetracarboxylic dianhydride and chloroindium phthalocyanine: limitations to long-term stability. Chem Mater 7:657–662
Radi A, Pradhan D, Sohn Y, Leung KT (2010) Nanoscale shape and size control of cubic, cuboctahedral, and octahedral Cu-Cu2O core-shell nanoparticles on Si(100) by one-Step, templateless, capping-agent-free electrodeposition. ACS Nano 4:1553–1560
Xu JS, Xue DF (2006) Five branching growth patterns in the cubic crystal system: a direct observation of cuprous oxide microcrystals. Acta Mater 55:2397–2406
Gao LZ, Zhuang J, Nie L, Zhang JB, Zhang Y, Gu N, Wang TH, Feng J, Yang DL, Perrett S, Yan XY (2007) Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat Nanotechnol 2:577–583
Ma M, Xie J, Zhang Y, Chen ZP, Gu N (2013) Fe3O4@Pt nanoparticles with enhanced peroxidase-like catalytic activity. Mater Lett 105:36–39
Wei H, Wang EK (2013) Nanomaterials with enzyme-like characteristics (nanozymes): next-generation artificial enzymes. Chem Soc Rev 42:6060–6093
Dong JL, Song L, Yin JJ, He WW, Wu YH, Gu N, Zhang Y (2014) Co3O4 Nanoparticles with multi-enzyme activities and their application in immunohistochemical assay. ACS Appl Mater Interface 6:1959–1970
Liu YP, Wang CW, Cai N, Long SH, Yu FQ (2014) Negatively charged gold nanoparticles as an intrinsic peroxidase mimic and their applications in the oxidation of dopamine. J Mater Sci 49:7143–7150. doi:10.1007/s10853-014-8422-x
Yin JF, Cao HQ, Lu YX (2012) Self-assembly into magnetic Co3O4 complex nanostructures as peroxidase. J Mater Chem 22:527–534
Liu X, Wang Q, Zhao HH, Zhang LC, Su YY, Lv Y (2012) BSA-templated MnO2 nanoparticles as both peroxidase and oxidase mimics. Analyst 137:4552–4558
Soh N, Kaneko S, Uozumi K, Ueda T, Kamada K (2014) Preparation of an enzyme/inorganic nanosheet/magnetic bead complex and its enzymatic activity. J Mater Sci 49:8010–8015. doi:10.1007/s10853-014-8508-5
Zhang JW, Zhang HT, Du ZY, Wang XQ, Yu SH, Jiang HL (2013) Water-stable metal–organic frameworks with intrinsic peroxidase-like catalytic activity as a colorimetric biosensing platform. Chem Commun 50:1092–1094
Zhang LL, Han L, Hu P, Wang L, Dong SJ (2013) TiO2 nanotube arrays: intrinsic peroxidase mimetics. Chem Commun 49:10480–10482
Xian YZ, Xian Y, Zhou LH, Wu FH, Ling Y, Jin LT (2007) Encapsulation hemoglobin in ordered mesoporous silicas: influence factors for immobilization and bioelectrochemistry. Electrochem Commun 9:142–148
Dou J, Zeng HC (2014) Integrated networks of mesoporous silica nanowires and their bifunctional catalysis-sorption application for oxidative desulfurization. ACS Catal 4:566–576
Acknowledgement
This work was financially supported by the National Natural Science Foundation of China (Grant Nos. 21376192, 21176200 and 81571809) and the Natural Science Foundation of Shaanxi Province (Grant No. 2015JM2063).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, G.L., Ma, P., Zhang, Y.F. et al. Synthesis of Cu2O nanowire mesocrystals using PTCDA as a modifier and their superior peroxidase-like activity. J Mater Sci 51, 3979–3988 (2016). https://doi.org/10.1007/s10853-015-9716-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-015-9716-3