Abstract
In this study, epoxy-cubic boron nitride composites are fabricated, and their dielectric response is investigated. They exhibit the same trend as epoxy composites reinforced with other filler types. Thus, at low filler concentrations, they exhibit a lower relative permittivity than neat epoxy. As the fill grade further increases, the relative permittivity of the composites increases as well. It is suggested that two competing mechanisms, namely polymer re-organization and water uptake, mainly determine the dielectric response of epoxy composites. The former is related to the decrease while the latter to the increase of the relative permittivity. The experimental results clearly verify that water uptake strongly depends on the filler concentration and not only affects the dielectric response of epoxy composites but also it is one of the main mechanisms which dictate their dielectric behavior.
Similar content being viewed by others
Introduction
The dielectric response of epoxy nanocomposites is undoubtedly one of the properties which give us convincing evidence that the introduction of fillers affects the structure of epoxy. There are a significant number of publications in which composites exhibit lower permittivity values compared to that of the base material despite the fact that fillers have a higher permittivity than the base polymer [1–8]. The decrease in permittivity of composites can be attributed to the influence of the particles on the polymer structure [9–11]. Thus, re-organization of the polymer matrix and immobilization of the polymer chains close to the particles are considered as a potential mechanism which leads to lower permittivity values.
Composites most of the time exhibit the lowest relative permittivity at low fill grades [1, 12–15]. There is a critical filler concentration above which the relative permittivity increases with the filler content. It was suggested in a previous study that the increase in relative permittivity is related to water uptake [16]. Based on two main mechanisms, i.e., polymer re-organization and water uptake, the relative permittivity of various epoxy nanocomposites was successfully modeled. Water uptake was considered to positively depend on the filler content mainly due to the particle hydrophilicity [17].
In this study, the dielectric response of epoxy-cubic boron nitride (E-cBN) composites is presented. These composites show the same trend (minimum value of relative permittivity at a low fill grade) as other epoxy composites, and there is a significant influence of water uptake. The water effect was minimized by thermal treatment or/and vacuum storage, but it could not be completely eliminated. The dielectric response is clearly influenced by water absorption even after treating the samples. Also, it is demonstrated that the impact of water uptake depends on the fill grade. This is depicted not only in the dielectric response but also in the thermogravimetric analyses (TGA) carried out on the composites. Finally, the dielectric response of the composites with cBN particles is compared to that of neat epoxy and epoxy-hexagonal boron nitride nanocomposites. The latter were analytically presented in a previous study [18].
Experimental
Materials
The base material used in this study was a type of epoxy resin consisting of bisphenol-A diglycidyl CY225 and anhydrite hardener HY225. Epoxy was reinforced with cBN particles with an average particle size of 150 nm (supplied by Plasma Chem). The particles were modified with 3 wt% of (3-Glycidyloxypropyl)trimethoxysilane (EPPS) to improve the adhesion between the polymer matrix and fillers. Prior to the modification, the particles were dried at 140 °C for several days and dispersed in ethanol by means of an ultrasonic bath for approximately an hour.
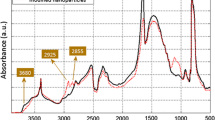
The reaction of hydroxyl groups on the surface of cBN with alkoxy groups of EPPS was evaluated with a diffuse reflectance infrared Fourier transform spectroscopy. Therefore, the spectra of the as-received and modified cBN particles are presented in Fig. 1. In the case of the as-received particles, the peak at 3680 cm−1 is ascribed to the free hydroxyl (OH) stretching vibrations of the B–OH surface groups. This peak is not observed in the spectrum of surface-modified particles indicating the successful reaction between EPPS and OH groups of the particles. Furthermore, in the case of modified particles, the peaks at 2925 and 2855 cm−1 verify the presence of EPPS. These peaks correspond to asymmetrical CH3 stretching and symmetrical CH2 stretching, respectively. Both of these groups are included in the chemical structure of EPPS.
Synthesis technique
An ex situ technique was used for the production of E-cBN composites. In this technique, modified particles are directly mixed with epoxy. The mixing of the two materials is performed with the use of speedmixer DAC 150.1 FVZ. Epoxy and dry modified particles are mixed for 15 min. Afterward, the proper amount of hardener is added to the mixture, and the material is mixed for 5 min. During the whole mixing process, zirconia balls with a diameter of 1.95 mm are used to achieve a better dispersion and distribution of particles. Finally, the mixture is degassed under vacuum and cast in aluminum molds. The material is cured at 140 °C for 20 h. Composites with four different filler concentrations of cBN were produced: E-cBN_02 (0.2 vol%), E-cBN_06 (0.6 vol%), E-cBN_1 (1 vol%), and E-cBN_5 (5 vol%).
Measuring and sample characterization devices
The dielectric response of the specimens was determined with the use of a fully automated Alpha-A dielectric analyzer from Novocontrol. The amplitude of the applied voltage was 3 V rms, and the samples were placed in a cryogenic cell in which the temperature was controlled in the range between −20 and 60 °C with steps of 20 °C, under nitrogen atmosphere. The accuracy of the measurements can be significantly affected by a number of error sources such as bad contacts and thickness variation [19]. In order to ensure a good contact between the samples and external electrodes, gold electrodes were deposited on both sides of the samples. Furthermore, the error in thickness determination was estimated by calculating twice the average thickness of a sample and comparing the difference in the results. Each average thickness was calculated based on five values. The error due to thickness variation is expected to be less than 1 %.
The distribution and dispersion of particles in the samples were evaluated with a scanning electron microscope (FEI Strata DB 235) which combines a field emission scanning electron column and a focused ion beam. The latter was used for milling the samples and preparing cross sections. Finally, TGA were performed using thermogravimetric analyzer TGA/SDTA 851e from Mettler Toledo.
Results
The results of the SEM analyses are presented in Figs. 2, 3, and 4. In all images, different locations of the samples are presented. The particles have been effectively dispersed, and they have been distributed in a quite homogeneous manner.
The results of the dielectric spectroscopy are presented in Figs. 5 and 6 where the relative permittivity and dielectric losses are presented as a function of frequency for different filler concentrations. Prior to the measurements, the samples were thermally treated in order to minimize/eliminate the effect of water uptake. The details of the thermal treatment of the samples are presented in Table 1.
The same trend, which was observed for other epoxy composites reinforced with different types of fillers, i.e., magnesium oxide [12], hexagonal boron nitride [20], aluminum oxide [12], and aluminum nitride [12], is also observed for E-cBN composites. Therefore, the composite with the lowest filler concentration, i.e., 0.2 vol%, shows the lowest relative permittivity which is lower than that of neat epoxy. As the filler concentration further increases, the composite relative permittivity increases as well. At 5 vol%, the composite exhibits much higher permittivity than neat epoxy.
The dielectric response of the composites is significantly influenced by water uptake. The effect of water uptake becomes more pronounced as the fill grade increases which reveals that the hydrophilic nature of the particles contributes to a great extent to the water absorption of the samples. The influence of water uptake is also depicted in the slope of the relative permittivity as a function of frequency (Fig. 5). It is observed that the slope of the relative permittivity becomes steeper for the composites with a higher filler loading. Thus, the composite with 0.2 vol% fill grade has the same slope as neat epoxy. For sample E-cBN_06, the slope of the relative permittivity becomes steeper at frequencies below 40 Hz while this point moves to 10 kHz for sample E-cBN_1. In the case of the composite with 5 vol% filler content, the relative permittivity exhibits a steeper slope in the whole frequency range, and the difference between the relative permittivity of neat epoxy and that of E-cBN_5 significantly increases as frequency decreases. The difference in slope is attributed to the significant water uptake and the high permittivity values of water, particularly at low frequencies [17].
As far as the imaginary part of the complex permittivity is concerned, it is observed that sample E-cBN_02 has lower losses at high frequencies which can be related to the suppression of the β-relaxation peak due to the immobilization of the polymer chains in the vicinity of the particles [12]. This is attributed to the chemical bonding between modified particles and matrix which results in the restructure of epoxy in the vicinity of the particles as schematically presented in Fig. 7. At lower frequencies, however, there is not much difference between neat epoxy and E-cBN_02 (Fig. 6). On the other hand, as the filler content increases, losses dramatically increase within the whole frequency range. Sample E-cBN_5 exhibits significantly higher losses than neat epoxy and an additional peak at nearly 1 kHz. This peak is related to water uptake, and a similar behavior has also been reported for epoxy-aluminum oxide nanocomposites [21].
Influence of the particle modification on the structure of epoxy [22]
Discussion
It is clear that water plays a drastic role in the dielectric response of composites. The intensity of the phenomenon becomes greater when the filler concentration increases, and thus, it is verified that particles have a hydrophilic nature although they are surface modified. In order to ensure the reproducibility of the results, two samples of the same batch were tested. Each sample was treated in a different way to minimize/eliminate the effect of water uptake. The fact that the results of two samples coincide regardless the way of treatment, ensures the high accuracy of the measurements. The treatment method of each sample is presented in Table 2, and all measurements are presented in Figs. 8 ,9, 10, 11, 12, 13, 14, and 15.
It is observed from the graphs that the composites are vulnerable to water uptake, and their dielectric response is significantly influenced by the presence of humidity. Thermal treatment under vacuum can help remove water from the samples. However, water remains in the samples and affects their dielectric response, especially at 1 and 5 vol% filler content. In the case of 5 vol% filler concentration, there is a dramatic influence of water uptake which is depicted both in the real and imaginary part of the complex permittivity. It seems impossible to get rid of the water influence as two samples of the same batch show an identical dielectric response although they were treated in a different way. Two factors can play an important role in this behavior, i.e., particle hydrophilicity and high particle density (3.45 g/cm3). The former is related to the adsorption of water, while the latter is related to the difficulty in removing water from the sample due to the high composite density.
The presence of water also affects the dielectric losses of a sample at lower temperatures. The losses of all E-cBN composites at −20 °C are presented in Figs. 16, 17, 18, and 19. It is clear that “wet” samples exhibit an additional peak which is located nearly at 150 Hz. This peak fades away when the samples are dry. Also, it is observed that the peak moves to higher frequencies when a larger amount of water has been absorbed. In Fig. 19, sample E-cBN_5 which had been exposed to ambient conditions (RH = 65 %, T = 22 °C) prior to the second measurement, shows a peak at 1 kHz while a relative dry sample with the same filler concentration has a peak close to 150 Hz. Although there is a significant reduction of the losses when sample E-cBN_5 is “dry,” the peak at 150 Hz is still pronounced due to the significant influence of water uptake.
The dielectric response of E-cBN composites is compared to the response of unfilled epoxy and another type of epoxy nanocomposites filled with hexagonal boron nitride (E-hBN) which was analyzed in a previous study [18]. The main goal is to verify that the additional peak at −20 °C is related to water uptake as unfilled epoxy is also susceptible to water uptake [23, 24]. Neat epoxy was treated in different ways as it is described in Table 3, and the effect of water uptake on the dielectric losses is presented in Fig. 20. It is observed that a dry sample exhibits almost no additional peak at 150 Hz. However, as the water content of the sample increases, due to the exposure to environmental conditions, the peak becomes more pronounced and moves to higher frequencies. It seems that 5 kHz is the maximum frequency at which this additional peak can occur. It is interesting to observe that the additional peak moves to a lower frequency (from 2.8 to nearly 1 kHz) after storing the sample under vacuum for a short period of time (Fig. 20: measurement 3 and measurement 4).
Furthermore, the behavior of E-hBN nanocomposites with 5 vol% (E-hBN_5) and 0.2 vol% (E-hBN_02) filler concentration with regard to water uptake was evaluated. Both samples were differently treated; the details for each sample are described in Table 4; and the respective results are presented in Figs. 21 and 22, respectively. It is observed that the additional peak, which occurs between 150 Hz and 5 kHz, is clearly related to the amount of water content.
Finally, the water content of E-cBN and E-hBN composites was evaluated by using TGA. The analyses were performed under helium atmosphere with a heating rate of 5 °C/min, and the first derivative of the weight loss is presented in Fig. 23. In the temperature range of 100–190 °C, the difference between neat epoxy and epoxy composites increases with the fill grade. The increasing difference can be attributed to the positive dependence of water content on the fill grade due to the particle hydrophilicity. Also, the results of TGA show that the difference between neat epoxy and E-cBN_5 is greater than the difference between neat epoxy and E-hBN_5. This difference is attributed to the larger amount of water inclusion for sample E-cBN_5, and it is clearly depicted in the dielectric response of E-cBN_5 and E-hBN_5. The former is significantly influenced by the presence of water, while the latter is slightly affected (Figs. 19, 22). The reason for the higher water content inside sample E-cBN_5 can be related to the greater hydroxyl availability of cBN particles which favors the adsorption of water [25].
Conclusions
The dielectric response of E-cBN composites was evaluated, and it was found that they show a similar behavior with other epoxy nanocomposites, namely epoxy-aluminum nitride, epoxy-magnesium oxide, epoxy-aluminum oxide, and epoxy-hexagonal boron nitride. At low filler concentrations, they exhibit a relative permittivity which is lower than that of neat epoxy. However, as the filler concentration further increases, the relative permittivity of the composites increases as well, surpassing that of neat epoxy.
Also, it has been experimentally shown that water uptake significantly affects the dielectric response of neat epoxy and epoxy composites. It was demonstrated that water uptake increases with the filler concentration, and both dielectric spectroscopy and TGA results verified the dependence of water content on the fill grade.
It is suggested that two main mechanisms, namely re-organization of the polymer matrix and water uptake, determine the dielectric response of epoxy composites. Surface modification of particles creates chemical bonds between the latter and matrix which result in the immobilization of the polymer chains in the vicinity of the particles. The created chemical bonds influence the structure of the polymer in the vicinity of the fillers by altering the amorphous structure of epoxy. On the other hand, water uptake significantly affects the dielectric response of composites. The composites with relatively high fill grades are affected in a more pronounced way than the samples with low filler concentrations (≤1 vol%) which means that particles, even if they are chemically treated, are susceptible to water adsorption. Water cannot be totally removed from the samples, especially when bound water is involved. Therefore, the decrease of the relative permittivity is attributed to the re-organization of the polymer matrix and immobilization of the polymer chains in the vicinity of the particles whereas the increase is related to the water uptake due to the hydrophilic nature of particles.
References
Kochetov R, Andritsch T, Morshuis PHF, Smit JJ (2012) Anomalous behaviour of the dielectric spectroscopy response of nanocomposites. IEEE Trans Dielectr Electr Insul 19:107–117
Heid TF, Freebody N, Frechette MF, Couderc H, Vanga C, David E, Vaughan AS (2013) Dielectric properties of epoxy/BN micro- and meso-composites: dielectric response at low and high electrical field strengths. IEEE Electrical Insulation Conference, pp 25–29
Singha S, Thomas MJ (2008) Permittivity and tan delta characteristics of epoxy nanocomposites in the frequency range of 1 MHz–1 GHz. IEEE Trans Dielectr Electr Insul 15:2–11
Maity P, Poovamma PK, Basu S, Parameswaran V, Gupta N (2009) Dielectric spectroscopy of epoxy resin with and without nanometric alumina fillers. IEEE Trans Dielectr Electr Insul 16:1481–1488
Iyer G, Gorur RS, Richert R, Krivda A, Schmidt LE (2011) Dielectric properties of epoxy based nanocomposites for high voltage insulation. IEEE Trans Dielectr Electr Insul 18:659–666
Singha S, Thomas MJ, Kulkarni A (2010) Complex permittivity characteristics of epoxy nanocomposites at low frequencies. IEEE Trans Dielectr Electr Insul 17:1249–1258
Krivda A et al (2012) Characterization of epoxy microcomposite and nanocomposite materials for power engineering applications. IEEE Electr Insul Mag 28:38–51
Ciuprina F, Plesa I, Notingher PV, Tudorache T, Panaitescu D (2008) Dielectric properties of nanodielectrics with inorganic fillers. IEEE Conference on Electrical Insulation and Dielectric Phenomena, pp 682–685
Roy M, Nelson JK, MacCrone RK, Schadler LS, Reed CW, Keefe R, Zenger W (2005) Polymer nanocomposite dielectrics—the role of the interface. IEEE Trans Dielectr Electr Insul 12:629–643
Todd MG, Shi FG (2003) Characterizing the interphase dielectric constant of polymer composite materials: effect of chemical coupling agents. J Appl Phys 94:4551–4557
Lewis TJ (2005) Interfaces: nanometric dielectrics. J Phys D 38:202–212
Kochetov R (2012) Thermal and electrical properties of nanocomposites, including material processing. PhD Dissertation, Delft University of Technology
Andritsch T (2010) Epoxy based nanocomposites for high voltage dc applications, synthesis, dielectric properties and space charge dynamics. PhD Dissertation, Delft University of Technology
Sarathi R, Sahu RK, Rajeshkumar P (2007) Understanding the thermal, mechanical and electrical properties of epoxy nanocomposites. Mater Sci Eng 445–446:567–578
Singha S, Thomas MJ (2008) Dielectric properties of epoxy nanocomposites. IEEE Trans Dielect Electr Insul 15:12–23
Tsekmes IA, Kochetov R, Morshuis PHF, Smit JJ (2014) A unified model for the permittivity and thermal conductivity of epoxy based composites. J Phys D 47:415502
Dervos CT, Mergos JA, Skafidas PD, Athanassopoulou MD, Vassiliou P (2009) Effect of water on permittivity of nanodielectrics exposed to the atmosphere. IEEE Trans Dielectr Electr Insul 16:1558–1565
Tsekmes IA, Kochetov R, Morshuis PHF, Smit JJ (2015) The role of particle distribution in the dielectric response of epoxy-boron nitride nanocomposites. J Mater Sci 50:1175–1186. doi:10.1007/s10853-014-8674-5
Kochetov R, Tsekmes IA, Morshuis PHF, Smit JJ (2014) Inaccuracies in the dielectric permittivity due to thickness variation. IEEE Electrical Insulation Conference, pp 55–58
Tsekmes IA, Kochetov R, Morshuis PHF, Smit JJ (2014) Evaluating the effect of particle distribution and dispersion on the dielectric response of boron nitride–epoxy nanocomposites. IEEE Electrical Insulation Conference, pp 329–332
Zhang C, Stevens GC (2008) The dielectric response of polar and non-polar nanodielectrics. IEEE Trans Dielectr Electr Insul 15:606–617
Andritsch T, Kochetov R, Morshuis PHF, Smit JJ (2011) Proposal of the polymer chain alignment model. IEEE Conference on Electrical Insulation and Dielectric Phenomena, pp 624–627
Maxwell IA, Pethrick RA (1983) Dielectric studies of water in epoxy resins. J Appl Polym Sci 28:2363–2379
Abdelkader AF, White JR (2005) Water absorption in epoxy resins: the effects of the crosslinking agent and curing temperature. J Appl Polym Sci 98:2544–2549
Tsekmes IA, Kochetov R, Morshuis PHF, Smit JJ, Iizuka T, Tatsumi K, Tanaka T (2014) How different fillers affect the thermal conductivity of epoxy composites. IEEE Conference on Electrical Insulation and Dielectric Phenomena, pp 647–650
Acknowledgements
This study was financially supported by Senter Novem in the framework of IOP-EMVT Project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Tsekmes, I.A., Morshuis, P.H.F., Smit, J.J. et al. The influence of interfaces and water uptake on the dielectric response of epoxy-cubic boron nitride composites. J Mater Sci 50, 3929–3941 (2015). https://doi.org/10.1007/s10853-015-8940-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-015-8940-1