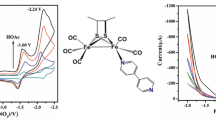
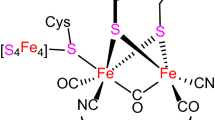
Abstract
In the presence of Me3NO in THF solution, the treatment of a diiron model complex (μ-dmedt)Fe2(CO)6 (dmedt = 2,3-butanedithiol) with carboxylic acid-functionalized (3-carboxypyridine) and amino-functionalized (4-aminopyridine) ligands afforded two new diiron model complexes, (μ-dmedt)Fe2(CO)5(3-COOHPy) (2) and (μ-dmedt)Fe2(CO)5(4-NH2Py) (3). Research on the influence of the 3-COOHPy and 4-NH2Py ligands upon the electrocatalytic characteristics of the diiron dithiolate core have been discussed in terms of spectroscopic and electrochemical findings. In the presence of HOAc in CH3CN solution, electrochemical studies show that complexes 2 and 3 can catalyse hydrogen evolution, with TOFs (the turnover frequencies) of 174.09 and 74.88 mol H2 (mol·cat)−1 h−1 cm−2 for complexes 2 and 3 at −1.96 V versus Fc+/Fc, respectively. The hydrogen evolution overpotentials of complexes 2 and 3 in the presence of HOAc in CH3CN solution were 0.73 and 0.82 V, respectively. Comparatively, complex 2 produces hydrogen at an overpotential 90 mV lower than complex 3 does. Complex 2 also has a better ability for electrocatalytic H2 production than complex 3. These studies provide a basic perception of the stereo-electronic characteristics related to the design for effective hydrogenase model complexes.
Graphical Abstract

Two new diiron model complexes, (μ-dmedt)Fe2(CO)5(3-COOHPy) (2), (μ-dmedt)Fe2(CO)5(4-NH2Py) (3), have been prepared, which served as efficient molecular electrocatalysts in the CH3CN solution, and the electrochemical studies show that complexes 2 and 3 can catalyse hydrogen evolution with TOFs of 174.09 and 74.88 mol H2 (mol·cat)−1 h−1 cm−2 for complexes 2 and 3 at −1.96 V versus Fc/Fc+, respectively.







Similar content being viewed by others
References
Fukuzumi S, Yamada Y, Suenobu T, Ohkubo K, Kotani H (2011) Catalytic mechanisms of hydrogen evolution with homogeneous and heterogeneous catalysts. Energy Environ Sci 4:2754–2766. doi:10.1039/c1ee01551f
Fukuzumi S, Yamada Y (2012) Catalytic activity of metal-based nanoparticles for photocatalytic water oxidation and reduction. J Mater Chem 22:24284–24296. doi:10.1039/c2jm32926c
Fukuzumi S, Yamada Y (2013) Shape- and size-controlled nanomaterials for artificial photosynthesis. ChemSusChem 10:1834–1847. doi:10.1002/cssc.201300361
Lubitz W, Ogata H, Rudiger O, Reijerse E (2014) Hydrogenases. Chem Rev 114:4081–4148. doi:10.1021/cr4005814
Pandey IK, Natarajan M, Kaur-Ghumaan S (2015) Hydrogen generation: aromatic dithiolate-bridged metal carbonyl complexes as hydrogenase catalytic site models. J Inorg Biochem 143:88–110. doi:10.1016/j.jinorgbio.2014.11.006
Peters JW, Lanzilotta WN, Lemon BJ, Seefeldt LC (1998) X-ray crystal structure of the Fe-only hydrogenase (CpI) from Clostridium pasteurianum to 1.8 angstrom resolution. Science 282: 1853–1858
Nicolet Y, Piras C, Legrand P, Hatchikian CE, Fontecilla-Camps JC (1999) Desulfovibrio desulfuricans iron hydrogenase: the structure shows unusual coordination to an active site Fe binuclear center. Structure 7:13–23. doi:10.1016/S0969-2126(99)80005-7
Armstrong FA (2013) Copying biology’s ways with hydrogen. Science 339:658–659. doi:10.1126/science.1233210
Feng YN, Xu FF, Chen RP, Wen N, Li Z, Du S (2012) Preparation, structures and electrochemical property of diiron dithiolate complexes with hydrophilic N-donor ligands. J Organomet Chem 717:211–217. doi:10.1016/j.jorganchem.2012.07.015
Zhang Y, Hu MQ, Wen HM, Si Y, Ma C, Chen C, Liu Q (2009) Terminal pyridine-N ligation at [FeFe] hydrogenase active-site mimic. J Organomet Chem 694:2576–2580. doi:10.1016/j.jorganchem.2009.03.050
Haley AL, Broadbent LN, McDaniel LS, Heckman ST, Hinkle CH, Gerasimchuk NN, Hershberger JC, Mebi CA (2016) [Fe-Fe] hydrogenase models: iron(I)-carbonyl clusters coupled to alpha- and para-toluenethiolate ligands. Polyhedron 114:218–224. doi:10.1016/j.poly.2015.12.031
Song LC, Zhu AG, Guo YQ (2016) Synthesis, characterization, and H/D exchange of μ-hydride-containing [FeFe]-hydrogenase subsite models formed by protonation reactions of (μ-TDT)Fe2(CO)4(PMe3)2 (TDT = SCH2SCH2S) with protic acids. Dalton Trans 4:55021–55029. doi:10.1039/c5dt04297f
Thomas CM, Rüdiger O, Liu T, Carson CE, Hall MB, Darensbourg MY (2007) Synthesis of carboxylic acid-modified [FeFe]-hydrogenase model complexes amenable to surface immobilization. Organometallics 26(16):3976–3984. doi:10.1021/om7003354
Zhao Z, Wang M, Dong W, Li P, Yu Z, Sun L (2009) Synthesis and characterization of carboxy-functionalized diiron model complexes of [FeFe]-hydrogenases: decarboxylation of Ph2PCH2COOH promoted by a diiron azadithiolate complex. J Organomet Chem 694(15):2309–2314. doi:10.1016/j.jorganchem.2009.03.039
Donovan ES, Nichol GS, Felton GAN (2013) Structural effects upon the durability of hydrogenase-inspired hydrogen-producing electrocatalysts: variations in the (μ-edt)[Fe2(CO)6] system. J Organomet Chem 726:9–13. doi:10.1016/j.jorganchem.2012.12.006
Wang Y, Zhang TY, Li B, Jiang S, Sheng L (2015) Electrochemical properties and catalytic reactivity of N-heterocyclic carbene-containing diiron complexes. RSC Adv 5:29022–29031. doi:10.1039/C4RA15150J.
Wang X, Zhang TY, Yang QS, Jiang S, Li B (2015) Synthesis and characterization of bio-inspired diiron complexes and their catalytic activity for direct hydroxylation of aromatic compounds. Berichte Der Deutschen Chemischen Gesellschaft, Eur J Inorg Chem 5:817–825. doi:10.1002/ejic.201402918
Si Y, Hu M, Chen C (2008) Diiron models for active site of FeFe-hydrogenase with aromatic thiolate bridges: structures and electrochemistry. C R Chimie 11:932–937. doi:10.1016/j.crci.2008.03.005
Ott S, Kritikos M, Åkermark B, Sun L (2003) Synthesis and structure of a biomimetic model of the iron hydrogenase active site covalently linked to a ruthenium photosensitizer. Angew Chem Int Ed 42:3285–3288. doi:10.1002/anie.200351192
Wang N, Wang M, Liu J, Jin K, Chen L, Sun L (2009) Preparation, facile deprotonation, and rapid H/D exchange of the μ-hydride diiron model complexes of the [FeFe]-hydrogenase containing a pendant amine in a chelating diphosphine ligand. Inorg Chem 48:11551–11558. doi:10.1021/ic901154m
Roy S, Groy TL, Jones AK (2013) Biomimetic model for [FeFe]-hydrogenase: asymmetrically disubstituted diiron complex with a redox-active 2,2′-bipyridyl ligand. Dalton Trans 42:3843–3853. doi:10.1039/c2dt32457a
Du H, Gu S, Liu R, Li CM (2015) Tungsten diphosphide nanorods as an efficient catalyst for electrochemical hydrogen evolution. J Power Sources 278:540–545. doi:10.1016/j.jpowsour.2014.12.095
Fiedler AT, Brunold TC (2005) Combined spectroscopic/computational study of binuclear Fe(I)-Fe(I) complexes: implications for the fully-reduced active-site cluster of Fe-only hydrogenases. Inorg Chem 44:1794–1809. doi:10.1021/ic048739n
Vannucci AK, Wang S, Nichol GS, Lichtenberger DL, Evans DH, Glass RS (2010) Electronic and geometric effects of phosphatriazaadamantane ligands on the catalytic activity of an [FeFe] hydrogenase inspired complex. Dalton Trans 39:3050–3056. doi:10.1039/b921067a
Song LC, Yang ZY, Bian HZ, Liu Y, Wang H, Liu XF, Hu QM (2005) Diiron oxadithiolate type models for the active site of iron-only hydrogenases and biomimetic hydrogen evolution catalyzed by Fe2(μ-SCH2OCH2S-μ)(CO)6. Organometallics 24:6126–6135. doi:10.1021/om0507373
Mejia-Rodriguez R, Chong D, Reibenspies JH, Soriaga MP, Darensbourg MY (2004) The hydrophilic phosphatriazaadamantane ligand in the development of H2 production electrocatalysts: iron hydrogenase model complexes. J Am Chem Soc 126:12004–12014. doi:10.1021/ja039394v
Chong D, Georgakaki IP, Mejia-Rodriguez R, Sanabria-Chinchilla J, Soriaga MP, Darensbourg MY (2003) Electrocatalysis of hydrogen production by active site analogues of the iron hydrogenase enzyme: structure/function relationships. Dalton Trans 21:4158–4163. doi:10.1039/B304283A
Greef R, Peat R, Peter LM, Pletcher D, Robinson J (1985) Instrumental methods in electrochemistry. Horwood Publishing, Southampton. doi:10.1002/bbpc.19860900614
Duan L, Wang M, Li P, Na Y, Wang N, Sun L (2007) Carbene–pyridine chelating 2Fe2S hydrogenase model complexes as highly active catalysts for the electrochemical reduction of protons from weak acid (HOAc). Dalton Trans 13:1277–1283. doi:10.1039/b616645h
Schwartz L, Ekström J, Lomoth R, Ott S (2006) Dynamic ligation at the first amine-coordinated iron hydrogenase active site mimic. Chem Commun 40:4206–4208. doi:10.1039/b608260b
Zhang X, Zhang T, Wang Y, Li B, Zhang G, Hai L, Jiang S (2016) Electrochemical catalysis investigation into the dynamic coordination properties of a pyridine-substituted [2Fe2S] model complex. Int J Hydrog Energy 41:22991–22996. doi:10.1016/j.ijhydene.2016.09.199
Zeng XH, Li ZM, Xiao ZY, Wang YW, Liu XM (2010) Using pendant ferrocenyl group(s) as an intramolecular standard to probe the reduction of diiron hexacarbonyl model complexes for the sub-unit of [FeFe]-hydrogenase. Electrochem Commun 12:342–345. doi:10.1016/j.elecom.2009.12.023
Windhager J, Rudolph M, Bräutigam S, Görls H, Weigand W (2007) Reactions of 1,2,4-trithiolane, 1,2,5-trithiepane, 1,2,5-trithiocane and 1,2,6-trithionane with nonacarbonyldiiron: structural determination and electrochemical investigation. Eur J Inorg Chem 2007:2748–2760. doi:10.1002/ejic.200700049
Felton GAN, Mebi CA, Petro BJ, Vannucci AK, Evans DH, Glass RS, Lichtenberger DL (2009) Review of electrochemical studies of complexes containing the Fe2S2, core characteristic of [FeFe]-hydrogenases including catalysis by these complexes of the reduction of acids to form dihydrogen. J Organomet Chem 694(17):2681–2699. doi:10.1016/j.jorganchem.2009.03.017
Zhang P, Wang M, Yang Y, Zheng D, Han K, Sun L (2014) Highly efficient molecular nickel catalysts for electrochemical hydrogen production from neutral water. Chem Commun 50:14153–14156. doi:10.1039/c4cc05511j
Cao JP, Zhao J X, Lu HX, Zhan SZ (2014) Synthesis and properties of a molybdenum Schiff base electrocatalyst for generating hydrogen from acetic acid. Transit Metal Chem 39:933–937. doi:10.1007/s11243-014-9878-x.
Fang T, Lu HX, Zhao JX, Zhan S, Lv Q (2015) A new copper(I)–triazenido electro-catalyst for catalyzing hydrogen evolution from acetic acid and water. J Mol Catal A 396:304–309. doi:10.1016/j.molcata.2014.10.008
Surawatanawong P, Tye JW, Darensbourg MY, Hall MB (2010) Mechanism of electrocatalytic hydrogen production by a di-iron model of iron-iron hydrogenase: a density functional theory study of proton dissociation constants and electrode reduction potentials. Dalton Trans 39:3093–3104. doi:10.1039/b925262b
Trautwein R, Almazahreh LR, Görlsa H, Weigan W (2015) Steric effect of the dithiolato linker on the reduction mechanism of [Fe2(CO)6{μ-(XCH2)2CRR’] hydrogenase models (X = S, Se)., Dalton Trans 44:18780–18794. doi:10.1039/c5dt01387a
Li Z, Zeng X, Niu Z, Liu X (2009) Electrocatalytic investigations of a tri-iron cluster towards hydrogen evolution and relevance to [FeFe]-hydrogenase. Electrochim Acta 54(13):3638–3644. doi:10.1016/j.electacta.2009.01.034
Sheldrick GM (1997) SHELXS-97, Program for crystal structure solution. University of Göttingen, Göttingen
Dolomanov O, Puschmann H (2007) Olex2: a quick prototyping framework for crystallographers. Acta Crystallogr 63:74–75. doi:10.1107/S0108767307098388.
Acknowledgements
This work was supported by the National Science Foundation of China (21276187), the Tianjin Science and Technology Innovation Platform Program (14TXGCCX00017) and the Tianjin Municipal Natural Science Foundation (16JCYBJC20800).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, X., Zhang, T., Li, B. et al. Synthesis and electrochemical properties of [FeFe]-hydrogenase model complexes with acid-functionalized or base-functionalized ligands. J Appl Electrochem 47, 583–591 (2017). https://doi.org/10.1007/s10800-017-1064-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-017-1064-3