Abstract
This paper demonstrates the fabrication of silver nanowires (AgNWs)/graphene oxide stacking structure for enhanced electrochemical performance. In this sandwich-type structure, the stratum of Ag nanowires acts as a highway bridge between the two layers of graphene oxide, where electrons can be transferred easily through this stacked network. The GO-AgNWs showed a specific capacitance of 251 Fg−1 at 10 mV s−1 and an energy density of 34.05 Wh kg−1 at 0.1 mA, indicating the positive synergistic effect of AgNW and GO. The obtained electrochemical characteristics are encouraging and found to be mainly attributed to an effective combination of graphene oxide and AgNWs.
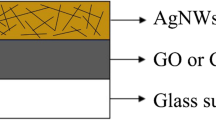
Graphical Abstract
Figure shows the steps involved in the development of the sandwich-like AgNW/GO nanostructure electrode.









Similar content being viewed by others
References
Frackowiak E (2007) Carbon materials for supercapacitor application. Phys Chem Chem Phys 9:1774–1785
Lee WH, Moon JH (2014) Monodispersed N-doped carbon nanospheres for supercapacitor application. ACS Appl Mater Interfaces 6:13968–13976
Liu Y, Shi Z, Gao Y, An W, Cao Z, Liu J (2016) Biomass-swelling assisted synthesis of hierarchical porous carbon fibers for supercapacitor electrodes.ACS Appl Mater Interfaces. doi:10.1021/acsami.5b11558
Yao Y, Ma C, Wang J, Qiao W, Ling L, Long D (2015) Rational design of high-surface-area carbon nanotube/microporous carbon core–shell nanocomposites for supercapacitor electrodes. ACS Appl Mater Interfaces 7:4817–4825
Li X, Wei B (2013) Supercapacitors based on nanostructured carbon. Nano Energy 2:159–173
Borchardt L, Oschatz M, Kaskel S (2014) Tailoring porosity in carbon materials for supercapacitor applications. Mater Horiz 1:157–168
Hummers WS, Offeman RE (1958) Preparation of graphitic oxide. J Am Chem Soc 80(6):1339–1339
Dimiev AM, Tour JM (2014) Mechanism of graphene oxide formation. ACS Nano 8:3060–3068
Pei S, Cheng HM (2012) The reduction of graphene oxide. Carbon 50:3210–3228
Xu B, Yue S, Sui Z, Zhang X, Hou S, Cao G, Yang Y (2011) What is the choice for supercapacitors: graphene or graphene oxide? Energy Environ Sci 4:2826–2830
Hu ZA, Wang YX, Xie YL, Yang YY, Zhang ZY, Wu HY (2010) Ag nanowires and its application as electrode materials in electrochemical capacitor. J Appl Electrochem 40:341–344
Yu Z, Li C, Abbitt D, Thomas J (2014) Flexible, sandwich-like Ag-nanowire/PE.:PSS nanopillar/MnO2 high performance supercapacitors. J Mater Chem A 2:10923–10929
Zhi J, Zhao W, Liu X, Chen A, Liu Z, Huang F (2014) Highly conductive ordered mesoporous carbon based electrodes decorated by 3D graphene and 1D silver nanowire for flexible supercapacitor. Adv Funct Mater 24:2013–2019
Yuksel R, Coskun S, Unalan HE (2016) Coaxial silver nanowire network core molybdenum oxide shell supercapacitor electrodes. Electrochim Acta 193:39–44
Yun YS, Kim DH, Kim B, Park HH, Jin HJ (2012) Transparent conducting films based on graphene oxide/silver nanowire hybrids with high flexibility. Synth Met 162:1364–1368
Shaw JE, Perumal A, Bradley DC, Stavrinou PN, Anthopoulos TD (2016) Nanoscale current spreading analysis in solution-processed graphene oxide/silver nanowire transparent electrodes via conductive atomic force microscopy. J Appl Phys 119:195501–195508
Chen R, Das SR, JeongC, Khan MR, Janes DB, AlamMA (2013) Co-percolating graphene-wrapped silver nanowire network for high performance, highly stable, transparent conducting electrodes. Adv Funct Mater. doi:10.1002/adfm.201300124
Meenakshi P, Karthick R, SelvarajM, Ramu S (2014) Investigations on reduced graphene oxide film embedded with silver nanowires as a transparent conducting electrode. Sol Energy Mater Sol Cells 128:264–269
Li SM, Wang YS, Hsiao ST, Liao WH, Lin CW, Yang SY, Tien HW, Ma CC, Hu CC (2015) Fabrication of a silver nanowire-reduced graphene oxide-based electrochemical biosensor and its enhanced sensitivity in the simultaneous determination of ascorbic acid, dopamine, and uric acid. J Mater Chem C 3:9444–9453
Khosrozadeh A, Darabi MA, Xing M, Wang Q (2016) Flexible electrode design: fabrication of freestanding polyaniline-based composite films for high-performance supercapacitors. ACS Appl Mater Interfaces 8:11379–11389
Zhang K, Zhang Y, Wang S (2013) Enhancing thermoelectric properties of organic composites through hierarchical nanostructures. Sci Rep 3:3448–3455
Mishra SK, Tripathi SN, Choudhary V, Gupta BD (2014) SPR based fibre optic ammonia gas sensor utilizing nanocomposite film of PMMA/reduced graphene oxide prepared by in situ polymerization. Sens Actuators B 199:190–200
Wilson NR, Pandey PA, Beanland R, Young RJ, Kinloch IA, Gong L, LiuZ, Suenaga K, Rourke JP, YorkS J, Sloan J, (2009) Graphene oxide: structural analysis and application as a highly transparent support for electron microscopy. ACS Nano 3:2547–2556
Xu B, Wu F, Wang F, Chen S, Cao GP, Yang YS (2006) Single-walled carbon nanotubes as electrode materials for supercapacitors. Chin J Chem 241505–1508
Bao Q, Bao S, Li CM, Qi X, Pan C, ZangJ, Lu Z, Li Y, Tang DY, Zhang S, Lian K (2008) Supercapacitance of solid carbon nanofibers made from ethanol flames. J Phys Chem C 112:3612–3618
Patil DS, Shaikh JS, Pawar SA, Devan RS, Ma YR, Moholkar AV, Kim JH, Kalubarme RS, Park CJ, Patil PS (2012) Investigations on silver/polyaniline electrodes for electrochemical supercapacitors. Phys Chem Chem Phys 14:11886–11895
Patil DS, Pawar SA, Devan RS, Gang MG, Ma YR, Kim JH, Patil PS (2013) Electrochemical supercapacitor electrode material based on polyacrylic acid/polypyrrole/silver composite. Electrochim Acta 105:569–577
Theophile N, Jeong HK (2017) Electrochemical properties of poly(vinyl alcohol) and graphene oxide composite for supercapacitor applications. Chem Phys Lett 669:125–129
BuI YY, Huang R (2017) Fabrication of CuO-decorated reduced graphene oxide nanosheets for supercapacitor applications. Ceram Int 43:45–50
Jeon H, Han JH, Yu DM, Lee JY, Kim TH, Hong YT (2017) Synthesis of mesoporous reduced graphene oxide by Zn particles for electrodes of supercapacitor in ionic liquid electrolyte. J Ind Eng Chem 45:105–110
Sliwak A, Grzyb B, Díez N, Gryglewicz G (2017) Nitrogen-doped reduced graphene oxide as electrode material for high rate supercapacitors. Appl Surf Sci 399:265–271
Dezfuli AS, Ganjali MR, Naderi HR (2017) Anchoring samarium oxide nanoparticles on reduced graphene oxide for high-performance supercapacitor. Appl Surf Sci 402:245–253
Nathan DMGT, Boby SJM (2017) Hydrothermal preparation of hematite nanotubes/reduced graphene oxide nanocomposites as electrode material for high performance supercapacitors. J Alloys Compd 700:67–74
Yuan D, Zeng J, Kristan N, Wang Y, Wang X (2009) Bi2O3 deposited on highly ordered mesoporous carbon for supercapacitors. Electrochem Commun 11:313–317
Patil DS, Shaikh JS, Dalavi DS, Kalagi SS, Patil PS (2011) Chemical synthesis of highly stable PVA/PANI films for supercapacitor application. Mater Chem Phys 128:449–455
Li X, ZangX, Li Z, Li X, Li P, Sun P, Lee X, Zhang R, Huang Z, Wang K, Wu D, Kang F, Zhu H (2013) Large-area flexible core–shell graphene/porous carbon woven fabric films for fiber supercapacitor electrodes. Adv Funct Mater 23:4862–4869
Patil DS, Pawar SA, Kim JH, Patil PS, Shin JC (2016) Facile preparation and enhanced capacitance of the Ag-PE.:PSS/polyaniline nanofiber network for supercapacitors. Electrochim Acta 213:680–690
Acknowledgements
This study was supported by the Korea Evaluation Institute of Industrial Technology (KEIT) Grant 10052824 funded by the Korea government (MOTIE).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Patil, D.S., Pawar, S.A., Patil, P.S. et al. Improved electrochemical performance of sandwich-like silver nanowires/graphene oxide nanostructure. J Appl Electrochem 47, 487–496 (2017). https://doi.org/10.1007/s10800-017-1053-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-017-1053-6