Abstract
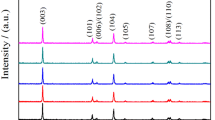
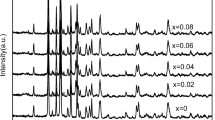
Zr-doped Li3V2–4x/3Zr x (PO4)3/C (x = 0, 0.05, 0.1, 0.15 or 0.2) composite cathode materials for lithium ion batteries had been prepared by a sol-gel method and characterized by X-ray diffraction, scanning electron microscopy, X-ray photoelectron spectroscopy, four-point probe measurements and electrochemical analyses. The cell volumes of the Zr-doped samples were larger than that of Li3V2(PO4)3, and Zr had an oxidation number of +4 in Zr-doped Li3V2–4x/3Zr x (PO4)3. The charge-transfer resistance of Li3V2(PO4)3/C was reduced and the reversibility was enhanced after Zr doping. All Zr-doped samples exhibited better electrochemical performance than pristine sample, and Li3V1.87Zr0.1(PO4)3/C composite displayed the highest capacity and the best cycle performance. The improved electrochemical performance mechanism for Zr-doped samples was discussed with respect to electronic structures using first-principle calculations. Zr doping did not significantly change the crystal structure of Li3V2(PO4)3, however, slight alterations in the lattice parameters and unit cell volume were observed. Li3V2(PO4)3 was affected by Zr doping at the V site with a narrower band gap as a result of the holes generated by activation of electrons to empty Zr states, leading to enhanced electronic conductivity of doped samples.










Similar content being viewed by others
References
Yamada A, Chung SC (2001) Crystal chemistry of the olivine-type Li(MnyFe1−y) PO4 and (MnyFe1−y)PO4 as possible 4 V cathode materials for lithium batteries. J Electrochem Soc 148(8):A960
Rui XH, Yesibolati N, Chen CH (2011) Li3V2(PO4)3/C composite as an intercalation-type anode material for lithium-ion batteries. J Power Sour 196:2279
Padhi AK, Nanjundaswamy KS, Goodenough JB (1997) Phospho-olivines as positive-electrode materials for rechargeable lithium batteries. J Electrochem Soc 144:1188
Whittingham MS (2004) Lithium batteries and cathode materials. Chem Rev 104:4271–4301
Chung SY, Bloking JT, Chiang YM (2002) Electronically conductive phospho-olivines as lithium storage electrodes. Nat Mater 1:123–125
Huang H, Yin S, Kerr T, Taylor N, Nazar LF (2002) Nanostructured composites: a high capacity, fast rate Li3V2(PO4)3/carbon cathode for rechargeable lithium batteries. Adv Mater 14:1525
Huang H, Faulkner T, Barker J, Saidi MY (2009) Lithium metal phosphates, power and automotive applications. J Power Sour 189:748–751
Wang L, Zhang LC, Lieberwirth I, Xu HW, Chen CH (2009) High capacity and excellent cyclability of vanadium (IV) oxide in lithium battery applications. Electrochem Commun 11:538–541
Saidi MY, Barker J, Huang H, Swoyer JL, Adamson G (2003) Performance characteristics of lithium vanadium phosphate as a cathode material for lithium-ion batteries. J Power Sour 121:266–272
Yao JH, Wei SS, Zhang PJ et al (2012) Synthesis and properties of Li3V2−x Ce x (PO4)3/C cathode materials for Li-ion batteries. J Alloys Compd 532:9
Dai CS, Chen ZY, Jin HZ, Hu XG (2010) Synthesis and performance of Li3(V1−x Mg x )2(PO4)3 cathode materials. J Power Sour 195:5775–5779
Zhang LL, Liang G, Peng G, Jiang Y, Fang H, Huang YH, Croft MC, Ignatov A (2013) Evolution of electrochemical performance in Li3V2(PO4)3/C composites caused by cation incorporation. Electrochim Acta 108:182–190
Son JN, Kim SH, Kim MC, Kim GJ, Aravindan V, Lee YG, Lee YS (2013) Superior charge-transfer kinetics of NASICON-type Li3V2(PO4)3 cathodes by multivalent Al3+ and Cl− substitutions. Electrochim Acta 97:210–215
Dang JX, Xiang F, Gu NY, Zhang RB, Mukherjee R, Il-Kwon Oh, Koratkar N, Yang ZY (2013) Synthesis and electrochemical performance characterization of Ce-doped Li3V2(PO4)3/C as cathode materials for lithium-ion batteries. J Power Sour 243:33–39
Chen YH, Zhao YM, An XN, Liu JM, Dong YZ, Chen L (2009) Preparation and electrochemical performance studies on Cr-doped Li3V2(PO4)3 as cathode materials for lithium-ion batteries. Electrochim Acta 54:5844–5850
Liu SQ, Li SC, Huang KL, Chen ZH (2007) Effect of doping Ti4+ on the structure and performances of Li3V2(PO4)3. Acta Phys Chim Sin 23:537–542
Yuan W, Yan J, Tang ZY, Sha Ou, Wang JM, Mao WF, Ma L (2012) Mo-doped Li3V2(PO4)3/C cathode material with high rate capability and long term cyclic stability. Electrochim Acta 72:138–142
Bai GL, Yang YF, Shao HX (2013) Synthesis and electrochemical properties of polyhedron-shaped Li3V2-x Sn x (PO4)3 as cathode material for lithium-ion batteries. J Electroanal Chem 688:98–102
Chen QQ, Qiao XC, Wang YB, Zhang TT, Yin WM, Liu L (2012) Electrochemical performance of Li3−x Na x V2(PO4)3/C composite cathode materials for lithium ion batteries. J Power Sour 201:267–273
Sato M, Ohkawa H, Yoshida K, Saito M, Uematsu K, Toda K (2000) Enhancement of discharge capacity of Li3V2(PO4)3 by stabilizing the orthorhombic phase at room temperature. Solid State Ionics 135:137–142
Ouyang CY, Shi SQ, Wang ZX et al (2004) First principles study of Li ion diffusion in LiFePO4. Phys Rev B 69:104303
Shi SQ, Ouyang CY, Xiong ZH et al (2005) First-principles investigation of the structural, magnetic, and electronic properties of olivine LiFePO4. Phys Rev B 71:144404
Jiang J, Ouyang CY, Li H, Wang ZX, Huang XJ, Chen LQ (2007) First-principles study on electronic structure of LiFePO4. Solid State Commun 143:144
Perdew JP, Chevary JA, Vosko SH et al (1992) Atoms, molecules, solids, and surfaces: applications of the generalized gradient approximation for exchange and correlation. Phys Rev B 46:6671
Monkhorst HJ, Pack J (1976) Integrand smooth (for non-metals): replace integral by sum over sampling points. Phys Rev B 13:5188
Ma B, Li Y, Su K (2009) Characterization of ceria-yttria stabilized zirconia plasma-sprayed coatings. Appl Sur Sci 255:7234–7237
Rui XH, Li C, Chen CH (2009) Synthesis and characterization of carbon-coated Li3V2(PO4)3 cathode materials with different carbon sources. Electrochim Acta 54:3374–3380
Qiao YQ, Tu JP, Wang XL, Gu CD (2012) The low and high temperature electrochemical performances of Li3V2(PO4)3/C cathode material for Li-ion batteries. J Power Sour 199:288
Chen QQ, Wang JM, Tang Z et al (2007) Electrochemical performance of the carbon coated Li3V2(PO4)3 cathode material synthesized by a sol-gel method. Electrochim Acta 52:5253
Kuang Q, Zhao YM, An XN, Liu JM, Dong YZ, Chen L (2010) Synthesis and electrochemical properties of Co-doped Li3V2(PO4)3 cathode materials for lithium-ion batteries. Electrochim Acta 55:1577
Cetinkaya T, Akbulut A, Guler MO, Akbulut H (2014) A different method for producing a flexible LiMn2O4/MWCNT composite electrode for lithium ion batteries. J Appl Electrochem 44:213
Ai DJ, Liu KY, Lu ZG, Zou MM, Zeng DQ, Ma J (2011) Aluminothermal synthesis and characterization of Li3V2−x Al x (PO4)3 cathode materials for lithium ion batteries. Electrochim Acta 56:2826
Yin SC, Strobel PS, Grondey H et al (2004) Charge ordering in lithium vanadium phosphates: electrode materials for lithium-ion batteries. Chem Mater 16:1456–1459
Acknowledgments
This work is supported by the Education Department Project of Heilongjiang Province (Grant No. 12531541) and the Dr. Fund of Heilongjiang Institute of Technology (Grant No. 2011BJ13).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xu, J., Chen, G., Zhang, H. et al. Electrochemical performance of Zr-doped Li3V2(PO4)3/C composite cathode materials for lithium ion batteries. J Appl Electrochem 45, 123–130 (2015). https://doi.org/10.1007/s10800-014-0782-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-014-0782-z