Abstract
Species invasions into ancient lakes are an important but little understood phenomenon. At ancient Lake Ohrid, a systematic assessment of invasive mollusc species using morphological and genetic data was conducted from 2003 to 2012. Two globally invasive gastropod species, Physa acuta and Ferrissia fragilis, have recently been discovered at 4 out of 386 sites. These sites are anthropogenically impacted. The invasive species co-occur with endemics. Phylogenetic analyses of populations from native and invaded ranges of both species confirmed their identities and provided insights into their invasion histories. Accordingly, P. acuta is genetically more diverse than F. fragilis. Both species are currently present in a considerable number of lakes on the Balkan Peninsula. Possible future trends in Lake Ohrid and the Balkans are discussed and further spread of both species is likely. Given the ongoing environmental change in Lake Ohrid, the number of observations of non-indigenous or other widespread species will probably rise in the coming years and such species and their impact on native species should be carefully monitored. Moreover, ancient lakes with recurrent invasions of alien species might serve as interesting model systems for the study of important topics of invasion biology.
Similar content being viewed by others
Introduction
Species invasions into ancient lakes are an important but little understood phenomenon. Some cases of invasive species in ancient lakes serve as textbook examples of ecological disasters following an introduction that happened decades ago (Goudswaard et al., 2008). The extent and possible impact of other introduction events became known only recently (e.g., Herder et al., 2012). This is particularly true for small benthic and inconspicuous invertebrate species. Molluscs, for example, are important components of the macrozoobenthos of all ancient lakes (e.g., Albrecht et al., 2012). However, very few recent gastropod invasions have been documented, among them Melanoides tuberculata O.F. Müller, 1774 in Lake Malawi (Genner et al., 2004) and Physa acuta Draparnaud, 1805 in Lake Titicaca (Albrecht et al., 2009a). Note that, the latter species is often attributed to the genus Haitia or Physella (but see Wethington & Lydeard, 2007). Interestingly, at about the same time P. acuta was introduced in the South American Lake Titicaca, it was also reported as invasive species in the European ancient Lake Ohrid (Albrecht et al., 2009b). In fact, this was the first record of an invasive mollusc species in Lake Ohrid, although the lake is historically among the best studied ancient lakes in terms of molluscs (Albrecht & Wilke, 2008; Hauffe et al., 2011). In particular, gastropods represent a major component of the macrozoobenthos of Lake Ohrid (Albrecht & Wilke, 2008) and historically 72 species of which 56 were considered as endemic (78 %) had been described from the lake (Radoman, 1985).
However, the introduction of non-indigenous species into the Lake has recently become of concern (Kostoski et al., 2010). The increase in number of invasive species has been attributed, in part, to the increasing anthropogenic pressure on the lake resulting in habitat transformations.
Given the recent discovery of the first invasive mollusc species in Lake Ohrid (i.e., P. acuta), and the “creeping biodiversity crisis” of the lake (Kostoski et al., 2010), the present study aims at a systematic assessment of invasive mollusc species in Lake Ohrid based on morphological and genetic data. The specific goals are:
-
(1)
A lake-wide assessment of invasive mollusc species in Lake Ohrid including habitat and mollusc community assessment; and
-
(2)
the reconstruction of the respective invasion histories in a global and regional Balkan context.
This is to the best of our knowledge the first comprehensive assessment of invasive molluscs in any of the ancient lakes worldwide. It can thus serve as case study and an outline for comparative discussions, potentially triggering similar studies in other ancient lakes.
Materials and methods
Sampling design
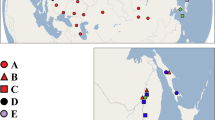
Materials from Lake Ohrid and its watershed were collected and faunal assessments were carried out during several field trips conducted between 2003 and 2012. Individuals were obtained by hand collecting from hard substrata in shallow waters or from stones and rocks lifted from depths to 5 m by snorkelling. Deeper parts of the littoral and sublittoral to 60 m were sampled using a dredge from small boats or from the research vessel of the Hydrobiological Institute in Ohrid (HBI). At Lake Ohrid, a total of 386 localities (285 in the lake proper, 101 in the basin; Fig. 1) have been surveyed for the presence of invasive species. The presence of invasive species was checked by morphological/anatomical examination and genotyping. For invasive species identified, materials from the respective type localities were included in this study (Table S1). Both habitat state relative to overall habitat conditions in Lake Ohrid (see Kostoski et al., 2010) and mollusc communities (using the methodology described in Hauffe et al., 2011) have been assessed for the localities from which invasive species had been identified.
Extensive field survey data from the Dessarete lakes (Albrecht & Wilke, 2008) and other lakes and occasionally other limnic systems of the Balkan Peninsula with repeated sampling in a total of 20 lakes, were analyzed and the respective faunistic literature was reviewed. We also conducted expert interviews in order to trace records of invasive species in Balkan lakes and thus to eventually assess the invasion histories of the invasive species reported from Lake Ohrid.
DNA sequencing
DNA was isolated from individual snails following the protocol described in Albrecht et al. (2004). Standard primers (Folmer et al., 1994) were used for amplifying a fragment of the cytochrome c oxidase subunit I (COI) gene with a target length of 655 bp (excluding 51 bp primer sequence). Sequences (forward and reverse) were generated either on a Long Read IR2 4200 sequencer (LI-COR) using the Thermo Sequenase Fluorescent Labeled Primer Cycle Sequencing kit (Amersham Pharmacia Biotech) or on an ABI 3730 XL sequencer (Life Technologies) using a Big Dye Terminator Kit (Life Technologies). All sequences could be unambiguously aligned using ClustalW implemented in BioEdit 7.0.8.0 (Hall, 1999).
Phylogenetic analyses
For each invasive species identified we conducted individual tree building analyses. First the best-fit model of sequence evolution was inferred for each data set based on the Akaike Information Criterion by conducting dynamical likelihood ratio tests in jModelTest 0.1.1 (Posada, 2008). Then we tested the degree of saturation for each dataset using the entropy-based approach of Xia et al. (2003) as implemented in DAMBE 5.2.9 (Xia & Lemey, 2009) with the input parameter for invariable sites taken from jModelTest.
Phylogenetic reconstructions using Bayesian inference were done with the software package MrBayes 3.1.2 (Huelsenbeck & Ronquist, 2001). During two independent runs, every 100th tree was sampled and individual analyses were terminated when final average standard deviations of split frequencies in MrBayes reached values of near or <0.01.
Results
Lake Ohrid assessment of invasive species
Prior to our survey, we identified 10 gastropod and 5 bivalve candidate invasive species (Table 1). The majority of these species (10 of 16) are known to be already present on the Balkan Peninsula. From these potentially invasive species, two were found to be currently present in Lake Ohrid: P. acuta and Ferrissia fragilis (Tryon, 1863). The former species occurred in 3 out of 386 surveyed localities (0.8 %), the latter one in 1 locality (0.3 %; Table 2).
The first thriving population of P. acuta was discovered in Lake Ohrid in May 2009. The population was found in the Ohrid Bay in close proximity to the channel leading to the Hydrobiological Institute Ohrid (41.10558°N, 20.80746°E; locality 1 in Fig. 1; also see Table S1) as well as in the channel itself (41.10362°N, 20.80946°E; locality 2 in Fig. 1). In 2010, the species has been recorded alive from a further location in the greater Ohrid Bay area (River Koselska; 41.12209°N, 20.77105°E; locality 3 in Fig. 1). All three places are impacted by human activities such as boating or fishing and contamination. The water quality is also worse than in most less influenced regions of Lake Ohrid. This is mostly due to sewage inflow. P. acuta has been found again during repeated monitoring conducted in 2010 and 2011.
Ferrissia fragilis was, for the first time, discovered at a site on the Albanian shore of Lake Ohrid opposite to Ohrid town (Lin Peninsula; 41.05138°N, 20.64190°E; locality 4 in Fig. 1, also see Table S1). It has hitherto been found exclusively at this place. This site is characterized by eutrophication caused by inflow of sewage and nutrient input from adjacent agricultural land.
In all four sites in which these invasive species were recorded, they co-occurred with endemic species, though at different degrees (Table 2). The most diverse mollusc community was found at locality 1 where P. acuta was associated with 12 other native and endemic species. P. acuta co-occurred with 5 other species in locality 2. In locality 3, however, only one additional (endemic) species was found. An unspecific littoral community (5 native and endemic species) characterized locality 4, where the population of Ferrissia thrives.
Phylogenetic analyses
The phylogenetic analyses of the Physa dataset included 114 specimens of a total of ten species, including 32 newly generated sequences from Balkan localities (for details see Table S1; Fig. 2). The Ferrissia dataset comprised two species with a total of 58 specimens of which 50 were obtained from GenBank (Fig. 3). Topotypic material for Physa acuta (River Garonne, France, AY282589) and Ferrissia wautieri (Lago di Mergozzo, Italy)—a species likely synonymous with F. fragilis—as well as new material of F. rivularis from the USA was included in the analyses.
Bayesian inference phylogram of Physidae based on 114 sequences of the COI gene under the GTR + I + G model. The outgroup taxa were a posteriori removed from the tree. They belong to the Hygrophila families Acroloxidae (Acroloxus lacustris), Planorbidae (Ancylus fluviatilis), and Lymnaeidae (Lymnaea stagnalis) used in a previous study (Albrecht et al., 2009a). Topotypes of Physa acuta are marked in bold. Clade assignment of Physidae follows Wethington and Lydeard (2007, Fig. 5a). Superscript numbers at particular localities refer to sampling sites listed in Table S1
Bayesian inference phylogram of Ferrissia spp. based on 58 sequences of the cytochrome c oxidase I gene under the TIM1 + G model. Topotypes of Ferrissia wautieri are marked in bold. The outgroup taxa were removed a posteriori. They belong to the Hygrophila families Acroloxidae, Planorbidae, and Lymnaeidae (from Albrecht et al., 2009a). Superscript numbers at particular localities refer to sampling sites listed in Table S1
Both under the assumption of a symmetrical as well as an asymmetrical tree, the Xia test indicated only little saturation in the Physa (GTR + I + G model) and Ferrissia (TIM1 + G model) data sets.
All morphologically identified Physa acuta specimens nested within the well-supported P. acuta clade (Bayesian posterior probability (BPP) 1.0, Fig. 2). A total of five different haplotypes were found in seven natural and two artificial lakes as well as four riverine systems on the Balkans. The Lake Ohrid haplotype (HTP1) is widespread on the Balkans and occurs also in specimens from Lakes Lysimachia, Pamvotis, Kerkini and Vegoritis, and the River Strymonas. Physa acuta populations from Lake Trichonis share a unique haplotype (HTP2), whereas there are two different haplotypes from the neighboring Lake Lysimachia (HTP1 and 3). HTP3 also occurs in Lake Kastraki. Lake Dojran specimens possessed haplotypes 4 (also River Gallikos) and 5. Haplotype 5 (Lakes Dojran, Kerkini, Volvi and River Strymonas) is closely related to haplotypes from the type locality of Physa acuta in France (Fig. 2; Table 1).
The Ferrissia specimens genotyped from lakes Ohrid, Lysimachia, Mergozzo, Kalodiki, and the Azores (San Miguel Island; GU391101; Raposeiro et al., 2011) constitute a single haplotype (Fig. 3). They cluster within a well-supported Ferrissia fragilis clade (BPP 1.0) together with representatives from North America, Europe, and Asia. Thus nominal European Ferrissia wautieri (Lake Mergozzo) is genetically (COI) not different from Ferrissia fragilis and therefore all European populations studied so far belong to the invasive F. fragilis.
Invasion histories
Physa acuta and Ferrissia fragilis are currently present in a considerable number of lakes on the Balkan Peninsula, including Lake Ohrid. From the major natural lakes of the Balkans considered here, the first record of P. acuta—to our knowledge—dates back to 1985 when it was reported from Lake Dojran (Stanković, 1985). Physa acuta today occurs in at least 13 (65 %) of the 20 studied lakes (Fig. 4). No Physa populations have been found in lakes Amvrakia, Petreo, and Zazari. Lakes Koronia and Pikrolimni were dried out during the field campaign in 2007, but no empty shells of Physa were found.
Ferrissia fragilis is at least present in ten of the surveyed lakes (50 %). All records were made after the year 2000, with the notable exception of Lake Vegoritis from where it already had been reported in 1962 (as F. wautieri; Schütt, 1985). All literature records of Ferrissia wautieri are considered here as representative of F. fragilis following the results of the genetic characterization of topotypic material of F. wautieri (see above). Details on first records and current status of both species in Balkan lakes can be found in the supplement (Tables S1, S2).
Discussion
Invasive species of Lake Ohrid
Hitherto, Lake Ohrid appeared to harbour fewer invasive species than other Balkan Lakes (Albrecht et al., 2009c, 2012). Given the presence of two globally invasive gastropod species reported here, it becomes obvious that highly impacted littoral parts such as the Ohrid Bay or areas near the Lin Peninsula have lately become prone to molluscs invasions. The quality of the mollusc fauna present in Lake Ohrid has thus changed significantly. Whereas hitherto the fauna was made up of widespread European or Balkan species and endemics (at various levels of endemicity; Hauffe et al., 2011), we now also see globally invasive species in the lake. Lake Ohrid thus shares the fate of Lake Malawi (Genner et al., 2004), Lake Titicaca (Albrecht et al., 2012), Lake Tahoe (Wittmann et al., 2012) and the Caspian Sea (Heiler et al., 2010) from which recently globally invasive molluscs species have been recorded. Of the total number of currently known gastropod species in Lake Ohrid (76), 56 are endemics to either the lake or its basin (73.7 %), 18 are widespread (23.7 %) and two are globally invasive species (2.6 %). Given our intensive surveys conducted during the last decade on the malacofauna of Lake Ohrid, we do consider the invasion of both species as, indeed, very recent events, especially in the case of the distinct P. acuta. The situation of F. fragilis might be a bit different in as much as it is a tiny limpet-like species. It could potentially be mistaken for an Acroloxus species, a genus of freshwater limpets present in Lake Ohrid with three to four species. In contrast to the long known presence of P. acuta in Europe, F. fragilis represents a case of a cryptic invader. Being also of North American origin, it was only recently shown to be conspecific with some populations of a Ferrissia wautieri (Walther et al., 2006). The latter species had been considered to be a European native by some authors (Falkner & Von Proschwitz, 1998; Raposeiro et al., 2011; Kadolski, 2012), but was questioned by others (Son, 2007; Marrone et al., 2011). No consensus could be reached due to the lack of modern data of topotypic material prior to this study.
It can only be speculated why Lake Ohrid has received both invasive species in a somewhat delayed manner compared to the other Balkan lakes (see below). Though slightly polluted at few sites, Lake Ohrid is still a relatively isolated oligotrophic lake (Matzinger et al., 2006) and thus far less frequented by migrating waterbirds compared to most other Balkan lakes. This is important, since waterbirds, especially Anatidae have been repeatedly suggested to be vectors of both P. acuta (e.g., Van Leeuwen et al., 2013) and F. fragilis (e.g., Kappes & Haase, 2012). It is impossible to predict whether (i) any of the two species will become permanent members of the malacocoenoses of Lake Ohrid and (ii) whether they will remain restricted to few affected sites. However, based on knowledge of other invaded regions (Bousset et al., 2004), there is no reason to assume that the populations might be of temporary nature only. Further monitoring must clarify the persistence of those populations and their impact on native mollusc communities. Whereas the competitive power of P. acuta has been shown repeatedly (e.g., Zukowski & Walker, 2009), no such observations are published for Ferrissia fragilis so far.
In the case of P. acuta, a new family of pulmonates is now present in Lake Ohrid. This is interesting since there are other families of gastropods (Bithyniidae) which are enigmatically absent though abundant otherwise on the Balkans (Glöer et al., 2007). However, there is data accumulating supporting far more pronounced seasonal or maybe long-term fluctuations in the presence or absence of species or even higher taxa in the lake.
Invasion histories
Most major freshwater habitat types are prone to P. acuta and F. fragilis invasions (Kappes & Haase, 2012; Van Leeuwen et al., 2013). Though earlier faunistic reviews of the Balkans reported P. acuta only from Croatia, Bulgaria (Jaeckel et al., 1957), and Greece (Käufle, 1930), recent comprehensive lists have recognized it also for Romania (Glöer & Sîrbu, 2006), Albania (Fehér & Erőss, 2009), and Montenegro (Glöer & Pešić, 2008). Reports for Macedonia, Serbia, and Bosnia and Herzegovina are scarce and apparently restricted to dam lakes or polluted running waters. Natural Balkan lakes, which have been subject to considerable malacological research, may serve as models to demonstrate an invasion trend obvious. P. acuta has been reported from Lake Skutari in 2002 by Dhora (2002) whereas a survey 5 years earlier (Jovanović, 1997) did no yield records of P. acuta. In all Balkan countries concerned and particularly in Greece, P. acuta and F. fragilis are present in many rivers and dam lakes (Albrecht & Wilke, unpublished data). This was not the case 25–30 years ago (e.g. Frank, 1983) when only scattered records were known from continental Greece. In Lake Pamvotis, no P. acuta was found in 1990 (Reischütz & Sattmann, 1990). In 2002, the first record was reported and the species presence has been confirmed ever since (Frogley & Preece, 2004, 2007). Lake Vegoritis was apparently free of P. acuta in 1985 (Schütt, 1985), new field data at the very same localities yielded vital populations 20 years later (Table S1). The situation in lakes Trichonis and Lysimachia was lately reviewed (Albrecht et al., 2009c). The first records of Physa acuta from these lakes date back to August 2002 (Reischütz & Reischütz, 2003). It is clear that some recent records in the Balkan lakes (except for Lake Ohrid, see above) may reflect rather delayed recognition due to more intense research efforts and absence in some lakes might likely be due to lower sampling intensity or simply a matter of chance.
Physid gastropods are known to be phenotypically plastic and thus some species are cryptic with misidentifications being a common problem (e.g., Anderson, 2003). Though the presence of other physid species in the lakes studied cannot be excluded completely, the demonstrated conspecifity of morphologically similar Physa spp. with P. acuta (Dillon et al., 2002), and the long invasion history in the Mediterranean is a strong argument in favor of exclusive P. acuta invasions into the lakes of the Balkans. Earlier reports of P. acuta in the fossil record of the Balkans (e.g., Schütt, 1987) predate the recognition of the species as a global invader and have to be treated with great caution.
For Ferrissia, our data have important implications for an ongoing discussion on the origin of European populations of this genus. All recent populations genotyped turned out to represent F. fragilis, an invader from North America (Walther et al., 2006, 2010; Marrone et al., 2011; Raposeiro et al., 2011). The conspecifity of this species with F. wautieri could not be tested prior to this study. This strongly supports the hypothesis that most if not all recent European populations are deriving from rather young invasion events. However, further research is necessary, especially in circum-Mediterranean regions. As already pointed out by Marrone et al. (2011), topotypic material from another species F. clessiniana (Jickeli, 1882) should be genotyped to finally clarify the confusing systematic situation of European populations of Ferrissia. Given the fact that F. fragilis is a cryptic invader, it is likely that the presence of this species in some Balkan lakes remains unrecognized. The fact, however, that only a single haplotype was found so far hints to a relatively recent invasion from a single or only few sources. The situation concerning Physa is different since we do have a higher haplotype diversity on the Balkan scale, with more than a single haplotype in some of the lakes. Such a pattern rather indicates multiple introductions from different sources and/or older or more diverse source populations.
Conclusions
Invasive molluscs such as P. acuta and F. fragilis can thrive in disturbed habitats and under fluctuating ecological circumstances. Given the current environmental situation in almost all Balkan lakes, the invasion success of at least P. acuta might be directly linked to human activities. It is unpredictable whether the presence of such invasive species is of only temporary nature and what competitive impact the invaders will have on native and particularly on the endemic gastropod faunas of Balkan lakes. At least for the time being, invasive molluscs species coexist with natives and endemics, as outlined in detail for Lake Ohrid. In fact, in this lake, there is not yet evidence for a competitive displacement. The general question in the Balkan lakes, however, is whether the habitat alteration (eutrophication etc.) is rather of superior importance in impacting native species as compared to the direct influence the invaders might have on other species. It is likely that these species will further spread all over the Balkans via active and/or passive dispersal. For the time being, only concerted international action plans can eventually enhance the environmental situation in the lakes affected and thus potentially slow down the further spread of P. acuta and F. fragilis. This is of particular importance for ancient Lake Ohrid where largely intact ecosystem conditions together with a relative remoteness have delayed the arrival of invasive molluscs species. Given the ongoing environmental change in Lake Ohrid, the number of observations of non-indigenous or other widespread species will probably rise in the coming years and such species and their relationship to native species should be carefully monitored. In most other lakes, the already reported decline and loss of endemic molluscs diversity will most likely continue and coincide with increasing reports of invasive molluscs species. Ancient lakes with their outstanding biodiversity and the recurrent invasion of alien species might serve as interesting model systems for the study of important topics of invasion biology such as the diversity–invasibility hypothesis.
References
Albrecht, C. & T. Wilke, 2008. Lake Ohrid: biodiversity and evolution. Hydrobiologia 615: 103–140.
Albrecht, C., T. Wilke, K. Kuhn & B. Streit, 2004. Convergent evolution of shell shape in freshwater limpets: the African genus Burnupia. Zoological Journal of the Linnean Society 140: 577–586.
Albrecht, C., C. Kroll & T. Wilke, 2009a. Invasion of ancient Lake Titicaca by the globally invasive Physa acuta (Gastropoda: Pulmonata: Hygrophila). Biological Invasions 11: 1821–1826.
Albrecht, C., K. Schreiber, T. Hauffe & T. Wilke, 2009b. Tracking biological invasions into ancient lakes: Physa acuta (Gastropoda: Hygrophila) on the Balkans. Review (Sbornik na rabotite) 42: 8–9.
Albrecht, C., T. Hauffe, K. Schreiber, S. Trajanovski & T. Wilke, 2009c. Mollusc biodiversity and endemism in the potential ancient Lake Trichonis, Greece. Malacologia 51: 357–375.
Albrecht, C., T. Hauffe, K. Schreiber & T. Wilke, 2012. Mollusc biodiversity in a European ancient lake system: lakes Prespa and Mikri Prespa in the Balkans. Hydrobiologia 682: 47–59.
Anderson, R., 2003. Physella (Costatella) acuta Draparnaud in Britain and Ireland – its taxonomy, origins and relationships to other introduced Physidae. Journal of Conchology 38: 7–21.
Bank, R. A., 2012. Fauna Europaea: Mollusca: Gastropoda. Fauna Europaea version 2.5. http://www.faunaeur.org.
Bousset, L., P.-Y. Henry, P. Sourrouille & P. Jarne, 2004. Population biology of the invasive freshwater snail Physa acuta approached through genetic markers, ecological characterization and demography. Molecular Ecology 13: 2023–2036.
DAISIE, 2009. Handbook of Alien Species in Europe. Springer, Dordrecht.
Dhora, D., 2002. The freshwater molluscs of Albania. In Dhora, D. (ed), Studies on the Molluscs of Albania. Camaj-Pipa, Shkodra: 115–130.
Dillon, R. T., A. R. Wethington, J. M. Rhett & T. P. Smith, 2002. Populations of the European freshwater pulmonate Physa acuta are not reproductively isolated from American Physa heterostropha or Physa integra. Invertebrate Biology 121: 226–234.
Falkner, G. & T. von Proschwitz, 1998. A record of Ferrissia (Pettancylus) clessiniana (Jickeli) in Sweden, with remarks on the identity and distribution of the European Ferrissia species. Journal of Conchology 36: 39–40.
Fehér, Z. & Z. P. Erőss, 2009. Checklist of the Albanian mollusc fauna. Schriften zur Malakozoologie 25: 22–38.
Folmer, O., M. Black, W. Hoeh, R. Lutz & R. Vrijenhoek, 1994. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Molecular Marine Biology and Biotechnology 3: 294–299.
Frank, C., 1983. Beitrag zur Molluskenfauna der östlichen Mittelmeerländer. Teil I: Bericht über eine Gastropodenausbeute aus Griechenland (Makedonien, Thrakien) vom Sommer 1981. Abhandlungen Staatliches Museum für Tierkunde Dresden 9: 69–80.
Frogley, M. R. & R. C. Preece, 2004. A faunistic review of the modern and fossil molluscan fauna from lake Pamvotis, Ioannina, an ancient lake in NW Greece: implications for endemism in the Balkans. In Griffith, H. I., B. Kryštufek & J. M. Reed (eds), Balkan Biodiversity. Kluwer Academic Publishers, Dordrecht: 243–260.
Frogley, M. R. & R. C. Preece, 2007. A review of the aquatic Mollusca from Lake Pamvotis, Ioannina, an ancient lake in NW Greece. Journal of Conchology 39: 271–295.
Genner, M. J., E. Michel, D. Erpenbeck, N. de Voogd, F. Witte & J. P. Pointier, 2004. Camouflaged invasion of Lake Malawi by an Oriental gastropod. Molecular Ecology 13: 2135–2141.
Glöer, P. & V. Pešić, 2008. The freshwater gastropods of the Skadar Lake with the description of Valvata montenegrina n. sp. (Mollusca, Gastropoda, Valvatidae). In: Pacićević, D. & M. Perreau (eds), Advances in the Studies of the Subterranean and Epigean Fauna of the Balkan Peninsula. Volume dedicated to the memory of Guido Nonvellier. Monography, Institute for Nature Conservation of Serbia: 341–348.
Glöer, P. & V. Pešić, 2013. A new freshwater snail genus (Hydrobiidae, Gastropoda) from Montenegro, with a discussion on gastropod diversity and endemism in Skadar Lake. ZooKeys 281: 69–90.
Glöer, P. & I. Sîrbu, 2006. New freshwater molluscs species found in the Romanian fauna. Heldia 6: 229–238.
Glöer, P., C. Albrecht & T. Wilke, 2007. Enigmatic distribution patterns of the Bithyniidae in the Balkan region (Gastropoda: Rissooidea). Mollusca 25: 13–22.
Goudswaard, K. P., F. Witte & E. F. Katunzi, 2008. The invasion of an introduced predator, Nile perch (Lates niloticus, L.) in Lake Victoria (East Africa): chronology and causes. Environmental Biology of Fishes 81: 127–139.
Hall, T. A., 1999. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Series 41: 95–98.
Hauffe, T., C. Albrecht, K. Schreiber, K. Birkhofer, S. Trajanovski & T. Wilke, 2011. Spatially explicit analysis of gastropod biodiversity in ancient Lake Ohrid. Biogeosciences 8: 175–188.
Heiler, K. C. M., N. Nahavandi & C. Albrecht, 2010. A new invasion into an ancient lake – the invasion history of the dreissenid mussel Mytilopsis leucophaeata (Conrad, 1831) and its first record in the Caspian Sea. Malacologia 53: 185–192.
Herder, F., U. K. Schliewen, M. F. Geiger, R. K. Hadiaty, S. M. Gray, J. S. McKinnon, R. P. Walter & J. Pfaender, 2012. Alien invasion in Wallace’s dreamponds: records of the hybridogenic “flowerhorn” cichlid in Lake Matano, with an annotated checklist of fish species introduced to the Malili Lakes system in Sulawesi. Aquatic Invasion 7: 521–535.
Huelsenbeck, J. P. & F. Ronquist, 2001. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 17: 754–755.
Jaeckel, S. G., W. Klemm & W. Meise, 1957. Die Land- und Süßwasser-Mollusken der nördlichen Balkanhalbinsel. Abhandlungen und Berichte des Museums Tierkunde und Völkerkunde zu Dresden 23: 141–205.
Jovanović, B. J., 1997. The Mollusca fauna of the Skadar Lake. Natural values and protection of Skadar Lake. Papers from a Symposium, Podgorica, November 8–9th, 1995: 263–277.
Kadolski, D., 2012. Nomenclatural comments on non-marine molluscs occurring in the British Isles. Journal of Conchology 41: 65–90.
Kappes, H. & P. Haase, 2012. Slow but steady: dispersal of freshwater molluscs. Aquatic Science 74: 1–14.
Käufle, F., 1930. Die schalentragenden Land- und Süßwassermollusken, Teil X. In Beier, M. (ed.), Zoologische Forschungsreise nach den Ionischen Inseln und dem Peloponnes. Sitzungsbericht der Mathematisch-Naturwissenschaftlichen Classe der Kaiserlichen Akademie der Wissenschaften Abteilung 1 139:161–188.
Kostoski, G., C. Albrecht, S. Trajanovski & T. Wilke, 2010. A freshwater biodiversity hotspot under pressure – assessing threats and identifying conservation needs for ancient Lake Ohrid. Biogeosciences 7: 3999–4015.
Marrone, F., S. Lo Brutto & M. Arculeo, 2011. Cryptic invasion in Southern Europe: the case of Ferrissia fragilis (Pulmonata: Ancylidae) Mediterranean populations. Biologia 66: 484–490.
Matzinger, A., Z. Spirkovski, S. Patceva & A. Wüest, 2006. Sensitivity of ancient Lake Ohrid to local anthropogenic impacts and global warming. Journal of Great Lakes Research 32: 158–179.
Posada, D., 2008. jModelTest: phylogenetic model averaging. Molecular Biology and Evolution 25: 1253–1256.
Radea, C., I. Louvrou & A. Economou-Amilli, 2008. First record of the New Zealand mud snail Potamopyrgus antipodarum J. E. Gray, 1843 (Mollusca: Hydrobiidae) in Greece – notes on its population structure and associated microalgae. Aquatic Invasions 3: 341–344.
Radoman, P., 1985. Hydrobioidea, a superfamily of Prosobranchia (Gastropoda), II Origin, Zoogeography, Evolution in the Balkans and Asia Minor. Monographs Institute of Zoology 1, Beograd.
Raposeiro, P. M., A. C. Costa & A. F. Martins, 2011. On the presence, distribution and habitat of the alien freshwater snail Ferrissia fragilis (Tyron, 1863) (Gastropoda: Planorbidae) in the oceanic islands of the Azores. Aquatic Invasion 6: 13–17.
Reischütz, A. & P. L. Reischütz, 2002. Hellenika pantoia, 1: Pyrgulidae (Gastropoda: Prosobranchia) aus dem Limni Pamvotis (Epirus, Griechenland). Nachrichtenblatt der Ersten Vorarlberger Malakologischen Gesellschaft 10: 1–4.
Reischütz, A. & P. L. Reischütz, 2003. Hellenika pantoia, 5: Zur Kenntnis der Molluskenfauna des Limni Trichonida und des Limni Lisimachia (Aitolien/Akarnien, Griechenland). Nachrichtenblatt der Ersten Vorarlberger Malakologischen Gesellschaft 11: 28–30.
Reischütz, A. & P. L. Reischütz, 2008. Hellenika pantoia, 18: Ein Beitrag zur Kenntnis der Molluskenfauna der böotischen Seen – Limni Yliki und Paralimni und ihre Umgebung (Zentral-Griechenland). Nachrichtenblatt der Ersten Vorarlberger Malakologischen Gesellschaft 15: 21–23.
Reischütz, P. L. & H. Sattmann, 1990. Beiträge zur Molluskenfauna des Epirus, 2. Annalen des Naturhistorischen Museums in Wien Serie B Botanik und Zoologie 91: 253–272.
Schütt, H., 1985. Die Mollusken des Vegoritis-Sees in Makedonien. Mitteilungen der Zoologischen Gesellschaft Braunau 12(13): 301–302.
Schütt, H., 1987. Limnische Mollusken aus älterem Quartär Makedoniens. Zoologische Mededelingen 61: 113–131.
Son, M. O., 2007. Native range of the zebra mussel and quagga mussel and new data on their invasion within the Ponto-Caspian region. Aquatic Invasions 2: 174–184.
Stanković, S., 1985. A contribution to the knowledge of gastropod fauna of Dojran Lake and the surrounding waters. Fragmenta Balcanica 13: 141–151.
Van Leeuwen, C. H. A., N. Huig, G. van der Velde, T. A. van Alen, C. A. M. Wagemarker, C. D. H. Sherman, M. Klaassen & J. Figuerola, 2013. How did this snail get here? Several dispersal vectors inferred for an aquatic invasive species. Freshwater Biology 58: 88–99.
Vardala-Theodorou, E., P. Polychronopoulos, P. Magiatis & A.-L. Skaltsounis, 2006. Contribution to the systematic and chemical study of malacofauna, of the Yliki-Paralimni lakes, Central Greece. Abstract, 10th Internationale Congress Zoogeographic & Ecology Greece Adjacent Regions, Patras, p. 122.
Walther, A. C., T. Lee, J. B. Burch & D. Ó. Foighil, 2006. Confirmation that the North American ancylid Ferrissia fragilis (Tryon, 1863) is a cryptic invader of European and East Asian freshwater ecosystems. Journal of Molluscan Studies 72: 318–321.
Walther, A. C., J. B. Burch & D. Ó. Foighil, 2010. Molecular phylogenetic revision of the freshwater limpet genus Ferrissia (Planorbidae: Ancylinae) in North America yields two species: Ferrissia (Ferrissia) rivularis and Ferrissia (Kincaidilla) fragilis. Malacologia 53: 25–45.
Wethington, A. R. & C. Lydeard, 2007. A molecular phylogeny of Physidae (Gastropoda: Basommatophora) based on mitochondrial DNA sequences. Journal of Molluscan Studies 73: 241–257.
Wilke, T., R. Schultheiß, C. Albrecht, N. Bornmann, S. Trajanovski & T. Kevrekidis, 2010. Native Dreissena freshwater mussels in the Balkans: in and out of ancient lakes. Biogeosciences 7: 3051–3061.
Wittmann, M. E., S. Chandra, J. E. Reuter, S. G. Schladow, B. C. Allen & K. J. Webb, 2012. The control of an invasive bivalve, Corbicula fluminea, using gas impermeable benthic barriers in a large natural lake. Environmental Management 49: 1163–1173.
Xia, X. & P. Lemey, 2009. Assessing substitution saturation with DAMBE. In Lemey, P., M. Salemi & A.-M. van Damme (eds), The Phylogenetic Handbook: A Practical Approach to DNA and Protein Phylogeny. Cambridge University Press, Cambridge: 615–630.
Xia, X., Z. Xie, M. Salemi, L. Chen & Y. Wang, 2003. An index of substitution saturation and its application. Molecular Phylogenetics and Evolution 26: 1–7.
Zukowski, S. & K. F. Walker, 2009. Freshwater snails in competition: alien Physa acuta (Physidae) and native Glyptophysa gibbosa (Planorbidae) in the River Murray, South Australia. Marine and Freshwater Research 60: 999–1005.
Acknowledgments
We are grateful to the colleagues who have supported our work in the Balkan countries, namely G. Kostoski, S. Trajanovski, D. Georgiev, A. Mogias, T. Kevrekidis, Z. Fehér, P. Glöer, V. Pešić. Special thanks go to the numerous students of the Wilke lab (Giessen) who took part in the field campaigns. We also particularly thank P. Reischütz for continuously sharing his knowledge on the Balkan malacofauna and for contributing valuable data to this paper. Z. Fehér is also gratefully acknowledged for providing important distributional records. C. Renker kindly donated Ferrissia specimens from Lake Kalodiki. We thank K. Heiler for helpful comments on an earlier version of this paper. The German Science Foundation (DFG AL 1076/3-1, WI 1902/8-2) supported our work on Lake Ohrid.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest editors: T. von Rintelen, R. M. Marwoto, G. D. Haffner & F. Herder / Speciation in Ancient Lakes – Classic Concepts and New Approaches
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Albrecht, C., Föller, K., Clewing, C. et al. Invaders versus endemics: alien gastropod species in ancient Lake Ohrid. Hydrobiologia 739, 163–174 (2014). https://doi.org/10.1007/s10750-013-1724-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-013-1724-1