Abstract
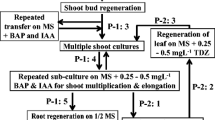
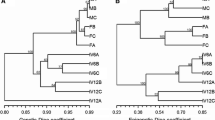
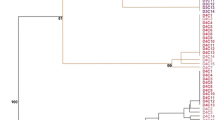
Gardenia jasminoides Ellis is an evergreen tropical plant and favorite to gardeners throughout the world. Several studies have documented that in vitro micropropagation can be used for clonal propagation of G. jasminoides Ellis, the efficiency remained low. In addition, no information is available on the genetic and epigenetic fidelity of the micropropagated plants. Here, we report on a simplified protocol for high efficient micropropagation of G. jasminoides Ellis cv. “Kinberly” based on enhanced branching of shoot-tips as explants. The protocol consisted of sequential use of three media, namely, bud-induction, elongation and root-induction. By using two molecular markers, amplified fragment length polymorphism (AFLP) and methylation sensitive amplified polymorphism (MSAP), we analyzed the genetic and DNA methylation pattern stability of 23 morphologically normal plants randomly taken from a sub-population (>100) of micropropagated plants originated from a single shoot-tip. We found that of >1,000 scored AFLP bands across the 23 micropropagated plants, no incident of genetic variation was detected. In contrast, of 750 scored MSAP bands, moderate but clear alteration in several DNA methylation patterns occurred in the majority of the 23 micropropagated plants. The changed methylation patterns involved both CG and CHG sites representing either hyper- or hypo-methylation, which occurred without altering the total methylation levels partly due to concomitant hyper- and hypo-methylation alterations. Our results indicated that epigenetic instability in the form of DNA methylation patterns can be susceptible to the in vitro micropropagation process for G. jasminoides Ellis, and needs to be taken into account in the process of large-scale commercial propagation of this plant.




Similar content being viewed by others
Abbreviations
- AFLP:
-
Amplified fragment length polymorphism
- 6-BAP:
-
6-Benzylaminopurine
- CTAB:
-
Cetyltrimethylammonium bromide
- GA3 :
-
Gibberellic acid
- IAA:
-
Indole-3-acetic acid
- IBA:
-
Indole-3-butyric acid
- MS:
-
Murashige and Skoog medium
- MSAP:
-
Methylation sensitive amplified polymorphism
- α-NAA:
-
1-Naphthaleneacetic basal acid
- RAPD:
-
Randomly amplified polymorphic DNA
- RFLP:
-
Restriction fragment length polymorphism
References
Ahuja MR (1987) In vitro propagation of poplar and aspen. Cell Tissue Cult For 3:207–223
Al-Juboory KH, Skirvin RM, Williams DJ (1998) Callus induction and adventitious shoot regeneration of Gardenia (Gardenia jasminoides Ellis) leaf explants. Sci Hortic 72:171–178
Bindiya K, Kanwar K (2003) Random amplified polymorphic DNA (RAPDs) markers for genetic analysis in micropropagated plants of Robinia pseudoacacia L. Euphytica 132:41–47
Cloutier S, Landry BS (1994) Molecular markers applied to plant tissue culture. In Vitro Cell Dev Biol Plant 30:32–39
Do GS, Seo BB, Ko JM, Lee SH, Pak JH, Kim IS, Song SD (1999) Analysis of somaclonal variation through tissue culture and chromosomal localization of rDNA sites by fluorescent in situ hybridization in wild Allium tuberosum and a regenerated variant. Plant Cell Tissue Organ Cult 57(2):113–119
Dong SK, Lee IS, Hyun DY, Jang CS, Song HS, Seo YW, Lee YI (2003) Detection of DNA instability induced from tissue culture and irradiation in Oryza sativa L. by RAPD analysis. J Plant Biotech 5(1):25–31
Fu XM, Zhou GX, Ge F, Lai XW, Zhu XL, Fan CS (2001) Survey of studies on Gardenia. Chin Wild Plant Resour 20(2):24–26
Gilman EF (1999) Gardenia jasminoides. Dissertation, University of Florida
Guo WL, Li YD, Gong L, Li FX, Dong YS, Liu B (2006) Efficient micropropagation of Robinia ambigua var. idahoensis (Idaho Locust) and detection of genomic variation by ISSR markers. Plant Cell Tissue Organ Cult 84:343–351
Hao YJ, Deng XX (2003) Genetically stable regeneration of apple plants from slow growt. Plant Cell Tissue Organ Cult 72:253–260
Kaeppler SM, Phillips RL (1993) Tissue culture-induced DNA methylation variation in maize. Proc Natl Acad Sci USA 90:8773–8776
Kubis SE, Castilho AMMF, Vershinin AV, Heslop-Harrison JS (2003) Retroelements, transposons and methylation status in the genome of oil palm (Elaeis guineensis) and the relationship to somaclonal variation. Plant Mol Biol 52(1):69–79
Larkin P, Scowcroft N (1981) Somaclonal variation—a novel source of variability from cell for plant improvement. Theor Appl Genet 60:197–214
Li YD, Guo WL, Liu XM, Shan XH, Li FX, Zhnag ZH, Liu B (2006) Efficient micropropagation of Japanese Photinia [Photinia glabra (Thunb.) Maxim.] retaining genetic and epigenetic stability. Propag Ornam Plants 6:149–155
Liang XM, Hu XM (2009) Shoot tip tissue culture and rapid propagation of Gardenia. J Anhui Agricult Sci 37:3949–3950
Lukens LN, Zhan S (2007) The plant genome’s methylation status and response to stress: implications for plant improvement. Curr Opin Plant Biol 10:317–322
McClelland M, Nelson M, Raschke E (1994) Effect of site-specific modification on restriction endonucleases and DNA modification methyltransferases. Nucleic Acids Res 22:3640–3659
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassay of tobacco tissue cultures. Physiol Plant 15:473–497
Polanco C, Ruiz ML (2002) AFLP analysis of somaclonal variation in Arabidopsis thaliana regenerated plants. Plant Sci 162:817–824
Predieri S (2001) Mutation induction and tissue culture in improving fruits. Plant Cell Tissue Organ Cult 64:185–210
Rahman MH, Rajora OP (2001) Microsatellite DNA somaclonal variation in micropropagated trembling aspen (Populus trimuloides). Plant Cell Rep 20:531–536
Reina-López GR, Simpson J, Ruiz-Herrera J (1997) Differences in DNA methylation patterns are detectable during the dimorphic transition of fungi by amplification of restriction polymorphism. Mol Gen Genomics 253:703–710
Ruiz ML, Rueda J, Peláez MI, Espino FJ, Candela M, Sendino AM, Vázquez AM (1992) Somatic embryogenesis plant regeneration and somaclonal variation in barley. Plant Cell Tissue Organ Cult 28:97–101
Sanal-Kumar P, Mathur VL (2004) Chromosomal instability in callus culture of Pisum sativum. Plant Cell Tissue Organ Cult 78:267–271
Soneji JR, Rao PS, Mhatre M (2002) Suitability of RAPD for analyzing spined and spineless variants and regenerants in pineapple (Ananas comosus L. Merr.). Plant Mol Biol Rep 20:307a–307i
Soniya EV, Banerjee NS, Das MR (2001) Genetic analysis of somaclonal variation among callus-derived plants of tomato. Curr Sci 80:9–10
Vendrame WA, Kochert GD, Wetzstein HY (1999) AFLP analysis of variation in pecan somatic embryos. Plant Cell Rep 18:853–857
Vendrame WA, Kochert GD, Wetzstein HY (2000) Field performance and molecular evaluations of pecan trees regenerated from somatic embryogenic cultures. J Am Soc Horticult Sci 125:542–546
Vos P, Hogers R, Bleeker M, Reijans M, Van De Lee T, Hornes M, Frijters A, Plot J, Peleman J, Kuiper M, Zabeau M (1995) AFLP: a new technique for DNA fingerprinting. Nucleic Acids Res 23:4407–4414
Wang PJ, Charles A (1991) Micropropagation through meristem culture. Biotechnol Agricult For 17:32–52
Wang YM, Dong ZY, Zhang ZJ, Lin XY, Shen Y, Zhou D, Liu B (2005) Extensive de Novo genomic variation in rice induced by introgression from wild rice (Zizania latifolia Griseb.). Genetics 170(4):1945–1956
Wilhelm E (2000) Somatic embryogenesis in oak (Quercus spp.). In vitro cellular and developmental. Biology Plant 36:349–357
Xiong LZ, Xu CG, Saghai Maroof MA, Zhang Q (1999) Patterns of cytosine methylation in an elite rice hybrid and its parental lines, detected by a methylation-sensitive amplification polymorphism technique. Mol Gen Genet 261(3):439–446
Yang H, Tabei Y, Kamada H, Kayano T, Takaiwa F (1999) Detection of somaclonal variation in cultured rice cells using digoxigenin-based random amplified polymorphic DNA. Plant Cell Rep 18:520–526
Acknowledgments
This study was supported by the National Natural Science Foundation of China (J0830627), and the Program of Introducing Talents of Discipline to Universities (B07017).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Ying Wu and Rui Wu have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Wu, Y., Wu, R., Zhang, B. et al. Epigenetic instability in genetically stable micropropagated plants of Gardenia jasminoides Ellis. Plant Growth Regul 66, 137–143 (2012). https://doi.org/10.1007/s10725-011-9637-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-011-9637-3