Summary
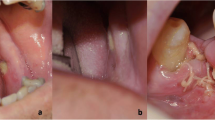
Purpose Bevacizumab, a monoclonal VEGF-A antibody, has been identified as an aetiology of osteonecrosis of the femoral head. Long exposure to anti VEGF therapy induced chronic hypoperfusion of normal tissues. Osteonecrosis is a musculo-skeletal disease secondary to cellular death of bone component mainly induced by corticosteroids, alcohol use, or connective tissue disorders. Methods The medical records of patients with metastatic colorectal carcinoma receiving Bevacizumab between January 2006 and November 2013 were retrospectively reviewed and we had looked for osteonecrosis. Every disorder of musculoskeletal mobility were examined by orthopaedist and evaluated by imaging. Results We report on osteonecrosis of humeral and femoral head in patient with metastatic colon adenocarcinoma receiving a long-term exposure to anti angiogenic based treatment (>6 months), lack of other factors predisposing to osteonecrosis. These observations, according to literature, suggests that long exposure to anti VEGF-A, Bevacizumab, promote bone hypoperfusion and may induced osteonecrosis either on the femoral head or the humeral head with an incidence of 4 out of 1000 patients. Conclusions With an incidence of 4 out of 1000 patients osteonecrosis is a rare side effect of anti-angiogenic agent. With the increasing utilisation and duration of exposure of anti-VEGF therapy some rare side effect due to chronic ischemia may appear. The clinician should be aware about uncommon symptoms.

Similar content being viewed by others
References
Lafforgue P (2006) Pathophysiology and natural history of avascular necrosis of bone. Joint Bone Spine 73:500–507
Mir O, Coriat R, Gregory T, Ropert S, Billemont B, et al. Avascular necrosis of the femoral head: a rare class-effect of anti-VEGF agents. Invest New Drugs 29: 716–718
Mancuso MR, Davis R, Norberg SM, O’Brien S, Sennino B et al (2006) Rapid vascular regrowth in tumors after reversal of VEGF inhibition. J Clin Invest 116:2610–2621
Arnold D, Andre T, Bennouna J, Sastre J, Osterlund PJ, et al (2012) Bevacizumab (BEV) plus chemotherapy (CT) continued beyond first progression in patients with metastatic colorectal cancer (mCRC) previously treated with BEV plus CT: Results of a randomized phase III intergroup study (TML study). J Clin Oncol 30, 2012 (suppl; abstr CRA3503) ASCO Annual Meeting
Bennouna J, Sastre J, Arnold D, Osterlund P, Greil R et al (2012) Continuation of bevacizumab after first progression in metastatic colorectal cancer (ML18147): a randomised phase 3 trial. Lancet Oncol 14:29–37
Grothey A, Cutsem EV, Sobrero A, Siena S, Falcone A, et al (2013) Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet
Van Cutsem E, Tabernero J, Lakomy R, Prenen H, Prausova J et al (2012) Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an Oxaliplatin-based regimen. J Clin Oncol 30:3499–3506
Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I et al (1981) A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther 30:239–245
Santos-Silva AR, Belizario Rosa GA, Castro Junior G, Dias RB, Prado Ribeiro AC et al (2013) Osteonecrosis of the mandible associated with bevacizumab therapy. Oral Surg Oral Med Oral Pathol Oral Radiol 115:e32–36
Lescaille G, Coudert AE, Baaroun V, Ostertag A, Charpentier E et al (2013) Clinical study evaluating the effect of bevacizumab on the severity of zoledronic acid-related osteonecrosis of the jaw in cancer patients. Bone 58C:103–107
Koczywas M, Cristea MC Osteonecrosis of the humeral head in a patient with non-small cell lung cancer receiving bevacizumab. J Thorac Oncol 6: 1960–1961.
Coriat R, Ropert S, Mir O, Billemont B, Chaussade S, et al. Pneumatosis intestinalis associated with treatment of cancer patients with the vascular growth factor receptor tyrosine kinase inhibitors sorafenib and sunitinib. Invest New Drugs 29: 1090–1093
Conflict of Interest
Dr. Tabouret, Dr. Gregory, Dr Dhooge, Dr Brezault, Dr Dréanic, Prof. Chaussade declare that they have no conflict of interest. Prof. Coriat reports personal fees from Amgen, Merck, Novartis, Roche and Sanofi-Aventis. Dr. Mir reports personal fees from Amgen, Astra-Zeneca, Bayer, Glaxo-Smith Kline, Novartis, Pfizer, Roche, and Servier.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tabouret, T., Gregory, T., Dhooge, M. et al. Long term exposure to antiangiogenic therapy, bevacizumab, induces osteonecrosis. Invest New Drugs 33, 1144–1147 (2015). https://doi.org/10.1007/s10637-015-0283-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-015-0283-x