Summary
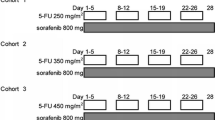
Background Sorafenib is the sole molecular-targeted agent showing a survival benefit in patients with advanced hepatocellular carcinoma (HCC). We evaluated the tolerability and effectiveness of a combination of S-1 with sorafenib in patients with advanced HCC. Methods S-1 was administered during days 1–14 and sorafenib was administered every day. This treatment was repeated every 21 days. In phase I, we determined the maximum tolerated dose (MTD) and dose-limiting toxicities (DLTs). The dose of each drug was planned as follows: cohort 1: S-1 48 mg/m2/day and sorafenib 400 mg/day, cohort 2a: S-1 48 mg/m2/day and sorafenib 800 mg/day, cohort 2b: S-1 64 mg/m2/day and sorafenib 400 mg/day, cohort 3: S-1 64 mg/m2/day and sorafenib 800 mg/day, and cohort 4: S-1 80 mg/m2/day and sorafenib 800 mg/day. In phase II, the patients were treated at the MTD to evaluate safety and efficacy. Results Nineteen patients were enrolled in phase I. One of the six patients in cohort 1 and one of the six patients in cohort 3 experienced DLT. None of the three patients in cohort 2a experienced DLT and three of the four patients in cohort 4 experienced DLT. Therefore, cohort 3 was considered the MTD. Subsequently, 26 patients were enrolled in phase II. The most common grade 3/4 toxicities were an increase of aspartate aminotransferase (38.5 %), thrombocytopenia (23.1 %), neutropenia (19.2 %), hyperbilirubinemia (15.4 %), an increase of alanine aminotransferase (15.4 %), hyponatremia (11.5 %), rash (11.5 %), and hypophosphatemia (11.5 %). Sudden death occurred in one patient (3.8 %). A patient (3.8 %) had a partial response, 15 (57.7 %) had stable disease, and 10 (38.5 %) had progressive disease. The median times to progression and overall survival were 2.4 and 10.5 months, respectively. Conclusion The MTD of S-1 and sorafenib in patients with advanced HCC was 64 mg/m2/day and 800 mg/day, respectively. This dose/regimen demonstrated substantial clinical activity among patients with advanced HCC.


Similar content being viewed by others
References
Ferlay J, Shin HR, Bray F et al (2010) Estimates of worldwide burden of cancer in 2008: Globocan 2008. Int J Cancer 127:2893–2917
Wilhelm SM, Carter C, Tang L et al (2004) Bay 43–9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res 64:7099–7109
Cheng AL, Kang YK, Chen Z et al (2009) Efficacy and safety of sorafenib in patients in the Asia-pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol 10:25–34
Llovet JM, Ricci S, Mazzaferro V et al (2008) Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 359:378–390
Takimoto CH, Awada A (2008) Safety and anti-tumor activity of sorafenib (nexavar) in combination with other anti-cancer agents: a review of clinical trials. Cancer Chemother Pharmacol 61:535–548
Shirasaka T, Shimamato Y, Ohshimo H et al (1996) Development of a novel form of an oral 5-fluorouracil derivative (S-1) directed to the potentiation of the tumor selective cytotoxicity of 5-fluorouracil by two biochemical modulators. Anticancer Drugs 7:548–557
Shirasaka T (2009) Development history and concept of an oral anticancer agent S-1 (TS-1): its clinical usefulness and future vistas. Jpn J Clin Oncol 39:2–15
Saif MW, Syrigos KN, Katirtzoglou NA (2009) S-1: a promising new oral fluoropyrimidine derivative. Expert Opin Investig Drugs 18:335–348
Furuse J, Okusaka T, Kaneko S et al (2010) Phase I/II study of the pharmacokinetics, safety and efficacy of S-1 in patients with advanced hepatocellular carcinoma. Cancer Sci 101:2606–2611
Zhai JM, Yin XY, Lai YR (2013) Sorafenib enhances the chemotherapeutic efficacy of S-1 against hepatocellular carcinoma through downregulation of transcription factor E2F-1. Cancer Chemother Pharmacol 71:1255–1264
Ogasawara S, Kanai F, Obi S et al (2011) Safety and tolerance of sorafenib in Japanese patients with advanced hepatocellular carcinoma. Hepatol Int 5:850–856
Lee SJ, Lee J, Park SH et al (2012) Phase 1 trial of S-1 in combination with sorafenib for patients with advanced hepatocellular carcinoma. Investig New Drugs 30:1540–1547
Furuse J, Ishii H, Nakachi K et al (2008) Phase I study of sorafenib in Japanese patients with hepatocellular carcinoma. Cancer Sci 99:159–165
Conflict of interest
Prof. Osamu Yokosuka received research grants from Bayer Healthcare. Yoshihiko Ooka, Tetsuhiro Chiba, Sadahisa Ogasawara, Kuniaki Arai, Eiichiro Suzuki, Akinobu Tawada, Tatsuya Yamashita, Fumihiko Kanai, and Shuichi Kaneko declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ooka, Y., Chiba, T., Ogasawara, S. et al. A phase I/II study of S-1 with sorafenib in patients with advanced hepatocellular carcinoma. Invest New Drugs 32, 723–728 (2014). https://doi.org/10.1007/s10637-014-0077-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-014-0077-6