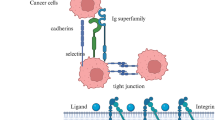
Abstract
Tumor cell metastasis is a complex, multi-step process that is a major cause of death and morbidity amongst cancer patients. Cell adhesion plays a critical role in the development of metastatic cancer, and it is mediated by interactions between receptors on the cell surface and ligands of the extracellular matrix or other surfaces. Therefore, inhibition of the cell adhesion process appears to be an effective method of preventing metastasis. This work describes a genetically engineered polypeptide with the potential to prevent cell adhesion and inhibit metastasis. We have found that the cell penetrating peptide Tat, fused with elastin-like polypeptide (ELP) inhibited adhesion, spreading, invasion and migration of SKOV-3 ovarian cancer cells in cell culture. Furthermore, we have also confirmed that Tat-ELP has anti-metastatic potential in an experimental ovarian cancer metastasis model in vivo, causing approximately 80% reduction in the tumor burden. Since cell attachment is an important step in tumor cell invasion and metastasis, these results suggest a novel role of Tat-ELP as a therapeutic intervention in cancer metastasis.






Similar content being viewed by others
Abbreviations
- CPP:
-
Cell penetrating peptide
- ELP:
-
Elastin-like polypeptide
- IP:
-
Intraperitoneal
- RFU:
-
Relative fluorescence units
- PBS:
-
Phosphate–buffered saline
- Tat:
-
CPP from HIV-1 transcription factor
References
Greenlee RT et al (2000) Cancer statistics, 2000. CA Cancer J Clin 50(1):7–33. doi:10.3322/canjclin.50.1.7
Vaccarello L et al (1995) Cytoreductive surgery in ovarian carcinoma patients with a documented previously complete surgical response. Gynecol Oncol 57(1):61–65. doi:10.1006/gyno.1995.1099
Geisler JP, Geisler HE (1995) Brain metastases in epithelial ovarian carcinoma. Gynecol Oncol 57(2):246–249. doi:10.1006/gyno.1995.1134
Niedbala MJ, Crickard K, Bernacki RJ (1985) Interactions of human ovarian tumor cells with human mesothelial cells grown on extracellular matrix. An in vitro model system for studying tumor cell adhesion and invasion. Exp Cell Resv 160(2):499–513. doi:10.1016/0014-4827(85)90197-1
Liotta LA, Steeg PS, Stetler-Stevenson WG (1991) Cancer metastasis and angiogenesis: an imbalance of positive and negative regulation. Cell 64(2):327–336. doi:10.1016/0092-8674(91)90642-C
Terranova VP, Hujanen ES, Martin GR (1986) Basement membrane and the invasive activity of metastatic tumor cells. J Natl Cancer Instvv 77(2):311–316
Iwamoto Y et al (1987) YIGSR, a synthetic laminin pentapeptide, inhibits experimental metastasis formation. Science 238(4830):1132–1134. doi:10.1126/science.2961059
Komazawa H et al (1993) Inhibition of tumor metastasis by Arg-Gly-Asp-Ser (RGDS) peptide conjugated with sulfated chitin derivative, SCM-chitin-RGDS. Clin Exp Metastasis 11(6):482–491. doi:10.1007/BF00054939
Massodi I, Bidwell GL 3rd, Raucher D (2005) Evaluation of cell penetrating peptides fused to elastin-like polypeptide for drug delivery. J Control Release 108(2–3):396–408. doi:10.1016/j.jconrel.2005.08.007
Brooks H, Lebleu B, Vives E (2005) Tat peptide-mediated cellular delivery: back to basics. Adv Drug Deliv Rev 57(4):559–577. doi:10.1016/j.addr.2004.12.001
Bidwell GL 3rd et al (2007) Development of elastin-like polypeptide for thermally targeted delivery of doxorubicin. Biochem Pharmacol 73(5):620–631. doi:10.1016/j.bcp.2006.10.028
Chilkoti A, Dreher MR, Meyer DE (2002) Design of thermally responsive, recombinant polypeptide carriers for targeted drug delivery. Adv Drug Deliv Rev 54(8):1093–1111. doi:10.1016/S0169-409X(02)00060-1
Massodi I, Raucher D (2007) A thermally responsive Tat-elastin-like polypeptide fusion protein induces membrane leakage, apoptosis, and cell death in human breast cancer cells. J Drug Target 15(9):611–622. doi:10.1080/10611860701502780
Meyer DE, Chilkoti A (2002) Genetically encoded synthesis of protein-based polymers with precisely specified molecular weight and sequence by recursive directional ligation: examples from the elastin-like polypeptide system. Biomacromolecules 3(2):357–367. doi:10.1021/bm015630n
Meyer DE, Chilkoti A (1999) Purification of recombinant proteins by fusion with thermally-responsive polypeptides. Nat Biotechnol 17(11):1112–1115. doi:10.1038/15100
Zhu N et al (2004) Melanoma cell migration is upregulated by tumour necrosis factor-alpha and suppressed by alpha-melanocyte-stimulating hormone. Br J Cancer 90(7):1457–1463. doi:10.1038/sj.bjc.6601698
Voura EB, Sandig M, Siu CH (1998) Cell-cell interactions during transendothelial migration of tumor cells. Microsc Res Tech 43(3):265–275. doi:10.1002/(SICI)1097-0029(19981101)43:3<265::AID-JEMT9>3.0.CO;2-Z
Zahm JM et al (1997) Cell migration and proliferation during the in vitro wound repair of the respiratory epithelium. Cell Motil Cytoskeleton 37(1):33–43. doi:10.1002/(SICI)1097-0169(1997)37:1<33::AID-CM4>3.0.CO;2-I
Wong MK, Gotlieb AI (1988) The reorganization of microfilaments, centrosomes, and microtubules during in vitro small wound reendothelialization. J Cell Biol 107(5):1777–1783. doi:10.1083/jcb.107.5.1777
Coomber BL, Gotlieb AI (1990) In vitro endothelial wound repair. Interaction of cell migration and proliferation. Arteriosclerosis 10(2):215–222
Zand L et al (2003) Differential effects of cellular fibronectin and plasma fibronectin on ovarian cancer cell adhesion, migration, and invasion. In Vitro Cellular & developmental biology. Animal 39(3–4):178–182. doi:10.1007/s11626-003-0013-0
Bidwell GL 3rd, Raucher D (2005) Application of thermally responsive polypeptides directed against c-Myc transcriptional function for cancer therapy. Mol Cancer Ther 4(7):1076–1085. doi:10.1158/1535-7163.MCT-04-0253
Fjeldstad K, Kolset SO (2005) Decreasing the metastatic potential in cancers–targeting the heparan sulfate proteoglycans. Curr Drug Targets 6(6):665–682. doi:10.2174/1389450054863662
Tarone G et al (1982) Cell surface molecules and fibronectin-mediated cell adhesion: effect of proteolytic digestion of membrane proteins. J Cell Biol 94(1):179–186. doi:10.1083/jcb.94.1.179
Saiki I et al (1990) Anti-metastatic and anti-invasive effects of polymeric Arg-Gly-Asp (RGD) peptide, poly(RGD), and its analogues. Jpn J Cancer Res 81(6–7):660–667
Brake DA, Debouck C, Biesecker G (1990) Identification of an Arg-Gly-Asp (RGD) cell adhesion site in human immunodeficiency virus type 1 transactivation protein, tat. J Cell Biol 111(3):1275–1281. doi:10.1083/jcb.111.3.1275
Dhawan S et al (1997) Human immunodeficiency virus-1-tat protein induces the cell surface expression of endothelial leukocyte adhesion molecule-1, vascular cell adhesion molecule-1, and intercellular adhesion molecule-1 in human endothelial cells. Blood 90(4):1535–1544
Benelli R et al (1998) Monocyte-derived dendritic cells and monocytes migrate to HIV-Tat RGD and basic peptides. AIDS) 12(3):261–268. doi:10.1097/00002030-199803000-00003
Mu Y et al (1999) Bioconjugation of laminin-related peptide YIGSR with polyvinyl pyrrolidone increases its antimetastatic effect due to a longer plasma half-life. Biochem Biophys Res Commun 264(3):763–767. doi:10.1006/bbrc.1999.1567
Kaneda Y et al (1995) Synthetic cell-adhesive laminin peptide YIGSR conjugated with polyethylene glycol has improved antimetastatic activity due to a longer half-life in blood. Invasion Metastasis 15(3–4):156–162
Mu Y et al (1999) Bioconjugation of laminin peptide YIGSR with poly(styrene co-maleic acid) increases its antimetastatic effect on lung metastasis of B16-BL6 melanoma cells. Biochem Biophys Res Commun 255(1):75–79. doi:10.1006/bbrc.1999.9930
Yamamoto Y, Tsutsumi Y, Mayumi T (2002) Molecular design of bioconjugated cell adhesion peptide with a water-soluble polymeric modifier for enhancement of antimetastatic effect. Curr Drug Targets 3(2):123–130. doi:10.2174/1389450024605427
Bidwell LG III, Raucher D (2005) Application of thermally responsive polypeptides directed against c-Myc transcriptional function for cancer therapy. Mol Cancer Ther 4(7):1076–1085. doi:10.1158/1535-7163.MCT-04-0253
Vogel BE et al (1993) A novel integrin specificity exemplified by binding of the alpha v beta 5 integrin to the basic domain of the HIV Tat protein and vitronectin. J Cell Biol 121(2):461–468. doi:10.1083/jcb.121.2.461
Acknowledgments
Grant support: This study was supported by the National Institute of Health R21 CA113813-01A2 and Wendy Will Case Foundation grant. We wish to thank Ms. Rowshan Begum and Ms. Leslie Robinson for technical assistance and critical reading of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Massodi, I., Bidwell, G.L., Davis, A. et al. Inhibition of ovarian cancer cell metastasis by a fusion polypeptide Tat-ELP. Clin Exp Metastasis 26, 251–260 (2009). https://doi.org/10.1007/s10585-009-9237-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10585-009-9237-z