Abstract
Four mesoporous SBA-15-immobilized N-heterocyclic carbene (NHC) palladium catalysts with different nitrogen ligands were synthesized. The activity and recyclability of the catalysts were investigated in Suzuki–Miyaura cross-coupling reaction of typical aryl chlorides and arylboronic acids. Bulky NHC moiety was essential for efficiency, while tethering of nitrogen ligands on both support and NHC group could dramatically improve recyclability of the catalysts. The strategy offers an alternative strategy for designing highly efficient and recyclable immobilized catalysts.
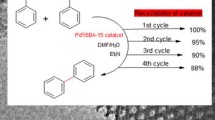
Graphical Abstract
Mesoporous silica-immobilized palladium catalysts with the bulky N-heterocyclic carbene and tethered “throw-away”nitrogen ligands were found to be highly efficient and recyclable for Suzuki–Miyaura coupling reaction of inactive aryl chlorides.








Similar content being viewed by others
References
Tamao K, Miyaura N (2002) Top Curr Chem 219:1
Negishi E (2007) Bull Chem Soc Jpn 80:233
Arpad M (2011) Chem Rev 111:2251
Littke AF, Fu GC (2002) Angew Chem Int Ed 41:4176
Miyaura N, Suzuki A (1995) Chem Rev 95:2457
Suzuki A (2011) Angew Chem Int Ed 50:6722
Yin L, Liebscher J (2007) Chem Rev 107:133
Dhara K, Sarkar K, Srimani D, Saha SK, Chattopadhyay P, Bhaumik A (2010) Dalton Trans 39:6395
Lamblin M, Nassar HL, Hierso JC, Fouquet E, Felpin FX (2010) Adv Synth Catal 352:33
Grushin VV, Alper H (1994) Chem Rev 94:1047
Arduengo AJ (1999) Acc Chem Res 32:913
Arduengo AJ, Harlow RL, Kline M (1991) J Am Chem Soc 113:361
Herrmann WA, Kocher C (1997) Angew Chem Int Ed 36:2162
Bourissou D, Guerret O, Gabbai FP, Bertrand G (2000) Chem Rev 100:39
Nelson DJ, Nolan SP (2013) Chem Soc Rev 42:6723
Kantchev EAB, O’Brien CJ, Organ MG (2007) Angew Chem Int Ed 46:2768
Herrmann WA, Reisinger CP, Spiegler M (1998) J Organomet Chem 557:93
Chen MT, Vicic DA, Turner ML, Navarro O (2011) Organometallics 30:5052
Gstottmayr CWK, Bohm VPW, Herdtweck E, Grosche M, Herrmann WA (2002) Angew Chem Int Ed 41:1363
Herrmann WA (2002) Angew Chem Int Ed 41:1290
Zapf A, Ehrentraut A, Beller M (2000) Angew Chem Int Ed 39:4153
Xu L, Chen W, Xiao J (2000) Organometallics 19:1123
Garrett CE, Prasad K (2004) Adv Synth Catal 346:889
Arvela RK, Leadbearter NE (2005) Org Lett 7:2101
Mubofu EB, Clark JH, Macquarrie DJ (2001) Green Chem 3:23
Park JC, Heo E, Kim A, Kim M, Park KH, Song H (2011) J Phys Chem C 115:15772
Kabalka GW, Pagni RM, Hair CM (1999) Org Lett 1:1423
Corma A, Garcia H, Leyva A (2005) J Mol Catal A 230:97
Hardy JE, Hubert S, Macquarrie DJ (2004) Green Chem 6:53
Mehnert CP, Weaver DW, Ying JY (1998) J Am Chem Soc 120:12289
Blaser HU, Indolese A, Schnyder A, Steiner H, Studer M (2001) J Mol Catal A 173:3
Kim JH, Kim JW, Shokouhimehr M, Lee YS (2005) J Org Chem 70:6714
Sayah R, Glegola K, Framery E, Dufaud V (2007) Adv Synth Catal 349:373
Qiu HL, Sarkar SM, Lee DH, Jin MJ (2008) Green Chem 10:37
Tyrrell E, Whiteman L, Williams N (2011) J Organomet Chem 696:3465
Ogasawara S, Kato S (2010) J Am Chem Soc 132:4608
Bhunia MK, Das SK, Pachfule P, Banerjee R, Bhaumik A (2012) Dalton Trans 41:1304
Byun JW, Lee YS (2004) Tetrahedron Lett 45:1867
Kim JH, Jun BH, Byun JW, Lee YS (2004) Tetrahedron Lett 45:5827
Lee DH, Kim JH, Jun BH, Kang H, Park JY, Lee YS (2008) Org Lett 10:1609
Yang H, Wang Y, Qin Y, Chong Y, Yang Q, Li G, Zhang L, Li W (2011) Green Chem 13:1352
Zhong R, Wang YN, Guo XQ, Chen ZX, Hou XF (2011) Chem Eur J 17:11041
Hou XF, Wang YN, Göttker-Schnetmann I (2011) Organometallics 30:6053
Guo XQ, Wang YN, Wang D, Cai LH, Chen ZX, Hou XF (2012) Dalton Trans 41:14557
Wang D, Guo XQ, Wang CX, Wang YN, Zhong R, Zhu XH, Cai LH, Gao ZW, Hou XF (2013) Adv Synth Catal 355:1117
Zhu XH, Cai LH, Wang CX, Wang YN, Guo XQ, Hou XF (2014) J Mol Catal A 393:134
Cao H, Zhu XH, Wang D, Sun Z, Deng Y, Hou XF, Zhao DY (2015) ACS Catal 5:27
Drew D, Doyle JR (1990) Inorg Synth 28:346
Vaghei RG, Hemmati S, Veisi H (2014) J Mol Catal A 393:240
Liu JP, Chen JB, Zhao JF, Zhao YH, Li L, Zhang HB (2003) Synthesis 17:2661
Gnanamgari D, Sauer ELO, Schley ND, Butler C, Incarvito CD, Crabtree RH (2009) Organometallics 28:321
Zhao D, Feng J, Huo Q, Melosh N, Frederickon GH, Chmelka BF, Stucky GD (1998) Science 279:548
Flahaut A, Baltaze JP, Roland S, Mangeney P (2006) J Organomet Chem 691:3498
Yang H, Han X, Li G, Wang Y (2009) Green Chem 11:1184
Meyer D, Taige MA, Zeller A, Hohlfeld K, Ahrens S, Strassner T (2009) Organometallics 28:2142
Liu G, Wang J, Huang T, Liang X, Zhang Y, Li H (2010) J Mater Chem 20:1970
Yang P, Zhao D, Margolese DI, Chmelka BF, Stucky GD, Galen D (1998) Nature 396:152
Tu T, Sun Z, Fang W, Xu M, Zhou Y (2012) Org Lett 14:4250
Karimi B, Enders D (2006) Org Lett 8:1237
Chen MT, Vicic DA, Chain WJ, Turner ML, Navarro O (2011) Organometallics 30:6770
Yang Y, Rioux RM (2014) Green Chem 16:3916
Shen A, Ni C, Cao Y-C, Zhou H, Song G-H, Ye X-F (2014) Tetrahedron Lett 55:3278
Kang T, Feng Q, Luo M (2005) Synlett 15:2305
Zhao Y, Zhou Y, Ma D, Liu J, Li L, Zhang Y, Zhang H (2003) Org Biomol Chem 1:1643
Stevens PD, Li G, Fan J, Yen M, Gao Y (2005) Chem Commun 35:4435
Acknowledgments
Financal support from the National Natural Science Foundation of China (Nos. 20871032, 20971026 and 21271047), ShanXi Science and Technology Department of China (Project No. MH2014-07), and General Administration of Quality Supervision, Inspection and Quarantine of China (Nos. 2015IK217)is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Zhang, JF., Wang, M., Guo, XQ. et al. Activity and Recyclability Improvement Through Adjusting the Tethering Strategy for Pd-Catalyzed Suzuki–Miyaura Coupling Reaction of Aryl Chlorides. Catal Lett 145, 2001–2009 (2015). https://doi.org/10.1007/s10562-015-1609-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-015-1609-1