Abstract
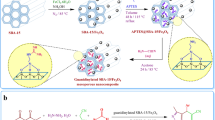
Magnetic α-Fe2O3–MCM-41 mesoporous material functionalized by dual acidic ionic liquid (DAIL) was prepared through a two-step approach as a novel powerful acidic catalyst which provides high ionic media for reactions, by means of post-surface grafting of the mesochannels of α-Fe2O3–MCM-41 materials with 3-sulfobutyl-1-(3-propyltrimethoxysilane)imidazolium hydrogen sulfate as DAIL. Mesoporous MCM-41 was simply packed on the surface of ferric oxide nanoparticles and DAIL was efficiently incorporated into the mesochannels of MCM-41 generating Lewis and Brönsted acidic sites. The catalyst was characterized by FT-IR, X-ray powder diffraction, HRTEM, vibrating sample magnetometer, X-ray energy diffraction spectra, thermogravimetric and differential thermal analyses, and N2 adsorption–desorption measurements. The synthesized hybrid acidic MCM-41 showed good catalytic performance in the one-pot synthesis of pyrimido[4,5-d]pyrimidine derivatives using 6-aminouracil, aldehyde, and urea or thiourea under solvent free conditions. Moreover, it was proved that under this condition, the use of such a hybrid material as a catalyst plays the role of rendering the reactions while neither the α-Fe2O3–MCM-41 nor the 3-methyl-1-(4-sulfonic acid)butylimidazolium hydrogen sulfate were able to promote this reaction.
Graphical Abstract













Similar content being viewed by others
References
Yadav GD, Sharma RV, Katole SO (2013) Ind Eng Chem Res 52:10133
Parangi TF, Wani BN, Chudasama UV (2013) Ind Eng Chem Res 52:8969
Corma A (2004) Catal Rev Sci Eng 46:369
Shylesh S, Schnemann V, Thiel WR (2010) Angew Chem Int Ed 49:3428
Molnar A, Rac B (2006) Curr Org Chem 10:1697
Hoffmann F, Cornelius M, Morell J, Frçba M (2006) Angew Chem 118:3290
Rostamizadeh S, Nojavan M, Aryan R, Isapoor E, Azad M (2013) J Mol Catal A 374–375:102
Galarneau A, Desplantier-Giscard D, Di Renzo F, Fajula F (2001) Catal Today 68:191
Rostamizadeh S, Shadjou N, Azad M, Jalali N (2012) Catal Commun 26:218
Wang C, Yang S, Chang H, Peng Y, Li J (2013) J Mol Catal A 376:13
Yang Q, Wei Z, Xing H, Ren Q (2008) Catal Commun 9:1307
Wang YY, Gong X, Wang Z, Dai LY (2010) J Mol Catal A 322:7
El-Moghazy SM, Diaa IA, Nagwa AM, Nahla FAH, El-Khouly AS (2011) Sci Pharm 79:429
Dabiri M, Arvin-Nezhad H, Khavasi HR, Bazgir A (2007) Tetrahedron 63:1770
Devi I, Borah HN, Bhuyan PJ (2004) Tetrahedron Lett 45:2405
Kidwai M, Singhal K, Kukreja S (2007) Z Naturforsch 62b:732
Gupta P, Gupta S, Sachar A, Kour D, Singh J, Sharma RL (2010) J Heterocyclic Chem 47:324
Graboyes H, Jaffe GE, Pachter IJ, Rosenbloom JP, Villani AJ, Wilson JW, Weinstock J (1968) J Med Chem 11:568
Zhang Q, Su H, Luo J, Wei Y (2012) Green Chem 14:201
Jahanbin B, Davoodnia A, Behmadi H, Tavakoli-Hoseini N (2012) Bull Korean Chem Soc 33:2140
Zhang Q, Luo J, Wei Y (2010) Green Chem 12:2246
Kassaee MZ, Masrouri H, Movahedi F (2011) Appl Catal A 359:28
Sahoo SK, Agarwal K, Singh AK, Polke BG, Raha KC (2010) Int J Eng Sci Technol 2:118
Elías VR, Oliva MI, Vaschetto EG, Urreta SE, Eimer GA, Silvetti SP (2010) J Magn Magn Mater 322:3438
Takahashi H, Li B, Sasaki T, Miyazaki C, Kajino T, Inagaki S (2001) Microporous Mesoporous Mater 44–45:755
Acknowledgments
The authors gratefully acknowledge K. N. Toosi University Research Council for partial financial support of this study.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Rostamizadeh, S., Nojavan, M., Aryan, R. et al. Dual Acidic Ionic Liquid Immobilized on α-Fe2O3–MCM-41 Magnetic Mesoporous Materials as the Hybrid Acidic Nanocatalyst for the Synthesis of Pyrimido[4,5-d]pyrimidine Derivatives. Catal Lett 144, 1772–1783 (2014). https://doi.org/10.1007/s10562-014-1330-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-014-1330-5