Abstract
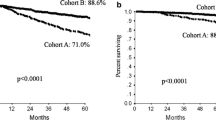
The prognosis of patients with stage II–III Human Epidermal growth factor Receptor 2 (HER2)-positive breast cancer has significantly improved since the addition of trastuzumab to (neo-)adjuvant chemotherapy. Several reports have shown that small (≤2 cm), node-negative, HER2-positive tumors have a relatively poor prognosis and these patients increasingly receive trastuzumab-based chemotherapy. We aimed to provide evidence for this approach in a population-based cohort. All T1N0M0 HER2-positive breast cancer patients diagnosed between 2006 and 2012 were identified from the Netherlands Cancer Registry. Patient, tumor, and treatment characteristics were recorded. Kaplan–Meier statistics were used for overall survival (OS) and breast cancer-specific survival (BCSS) estimations overall and in T1a, T1b, and T1c tumors separately. Cox regression analyses were performed to account for imbalances in baseline characteristics between treated and untreated patients. A total of 3512 patients were identified: 385 with T1a, 800 with T1b, and 2327 with T1c tumors. Forty-five percent of patients received chemotherapy and/or trastuzumab: 92 % received both. Chemotherapy and/or trastuzumab significantly improved 8-year OS (95 vs. 84 %; hazard ratio [HR] 0.29; 95 % confidence interval [CI] 0.21–0.41, P < 0.001). The effect remained significant in multivariable analyses (HR 0.35; 95 % CI 0.23–0.52, P < 0.001). BCSS was also improved with systemic treatment in univariable (96 vs. 92 %; HR 0.41; 95 % CI 0.27–0.63, P < 0.001) and multivariable analyses (HR 0.31; 95 % CI 0.19–0.53, P < 0.001). Treatment effect on OS and BCSS was similar in T1a, T1b, and T1c tumors. Chemotherapy and/or trastuzumab improves OS and BCSS and can be considered in all patients with small node-negative HER2-positive breast cancer.



Similar content being viewed by others
Abbreviations
- BCSS:
-
Breast cancer-specific survival
- CI:
-
Confidence interval
- DFS:
-
Disease-free survival
- DRFS:
-
Distant recurrence-free-survival
- ER:
-
Estrogen receptor
- HER2:
-
Human epidermal growth factor receptor 2
- HR:
-
Hazard ratio
- HR:
-
Hormone receptor
- IQR:
-
Interquartile range
- ITCs:
-
Isolated tumor cells
- NCR:
-
Netherlands Cancer Registry
- OS:
-
Overall survival
- PR:
-
Progesterone receptor
References
Joensuu H, Bono P, Kataja V, Alanko T, Kokko R, Asola R, Utriainen T, Turpeenniemi-Hujanen T, Jyrkkio S, Moykkynen K, Helle L, Ingalsuo S, Pajunen M, Huusko M, Salminen T, Auvinen P, Leinonen H, Leinonen M, Isola J, Kellokumpu-Lehtinen PL (2009) Fluorouracil, epirubicin, and cyclophosphamide with either docetaxel or vinorelbine, with or without trastuzumab, as adjuvant treatments of breast cancer: final results of the FinHer Trial. J Clin Oncol 27:5685–5692. doi:10.1200/JCO.2008.21.4577
Slamon D, Eiermann W, Robert N, Pienkowski T, Martin M, Press M, Mackey J, Glaspy J, Chan A, Pawlicki M, Pinter T, Valero V, Liu M-C, Sauter G, von Minckwitz G, Visco F, Bee V, Buyse M, Bendahmane B, Tabah-Fisch I, Lindsay M-A, Riva A, Crown J (2011) Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med 365:1273–1283. doi:10.1056/NEJMoa0910383
Perez EA, Romond EH, Suman VJ, Jeong JH, Sledge G, Geyer CE Jr, Martino S, Rastogi P, Gralow J, Swain SM, Winer EP, Colon-Otero G, Davidson NE, Mamounas E, Zujewski JA, Wolmark N (2014) Trastuzumab plus adjuvant chemotherapy for human epidermal growth factor receptor 2-positive breast cancer: planned joint analysis of overall survival from NSABP B-31 and NCCTG N9831. J Clin Oncol 32:3744–3752. doi:10.1200/JCO.2014.55.5730
Goldhirsch A, Gelber RD, Piccart-Gebhart MJ, de Azambuja E, Procter M, Suter TM, Jackisch C, Cameron D, Weber HA, Heinzmann D, Dal Lago L, McFadden E, Dowsett M, Untch M, Gianni L, Bell R, Kohne CH, Vindevoghel A, Andersson M, Brunt AM, Otero-Reyes D, Song S, Smith I, Leyland-Jones B, Baselga J, Herceptin Adjuvant Trial Study Team (2013) 2 years versus 1 year of adjuvant trastuzumab for HER2-positive breast cancer (HERA): an open-label, randomised controlled trial. Lancet 382:1021–1028. doi:10.1016/S0140-6736(13)61094-6
Gonzalez-Angulo AM, Litton JK, Broglio KR, Meric-Bernstam F, Rakkhit R, Cardoso F, Peintinger F, Hanrahan EO, Sahin A, Guray M, Larsimont D, Feoli F, Stranzl H, Buchholz TA, Valero V, Theriault R, Piccart-Gebhart M, Ravdin PM, Berry DA, Hortobagyi GN (2009) High risk of recurrence for patients with breast cancer who have human epidermal growth factor receptor 2-positive, node-negative tumors 1 cm or smaller. J Clin Oncol 27:5700–5706. doi:10.1200/JCO.2009.23.2025
Livi L, Meattini I, Saieva C, Franzese C, Di Cataldo V, Greto D, Franceschini D, Scotti V, Bonomo P, Nori J, Sanchez L, Vezzosi V, Bianchi S, Cataliotti L, Biti G (2012) Prognostic value of positive human epidermal growth factor receptor 2 status and negative hormone status in patients with T1a/T1b, lymph node-negative breast cancer. Cancer 118:3236–3243. doi:10.1002/cncr.26647
Theriault RL, Litton JK, Mittendorf EA, Chen H, Meric-Bernstam F, Chavez-Macgregor M, Morrow PK, Woodward WA, Sahin A, Hortobagyi GN, Gonzalez-Angulo AM (2011) Age and survival estimates in patients who have node-negative T1ab breast cancer by breast cancer subtype. Clin Breast Cancer 11:325–331. doi:10.1016/j.clbc.2011.05.002
Joensuu H, Isola J, Lundin M, Salminen T, Holli K, Kataja V, Pylkkanen L, Turpeenniemi-Hujanen T, von Smitten K, Lundin J (2003) Amplification of erbB2 and erbB2 expression are superior to estrogen receptor status as risk factors for distant recurrence in pT1N0M0 breast cancer: a nationwide population-based study. Clin Cancer Res 9:923–930
Chia S, Norris B, Speers C, Cheang M, Gilks B, Gown AM, Huntsman D, Olivotto IA, Nielsen TO, Gelmon K (2008) Human epidermal growth factor receptor 2 overexpression as a prognostic factor in a large tissue microarray series of node-negative breast cancers. J Clin Oncol 26:5697–5704. doi:10.1200/JCO.2007.15.8659
Vaz-Luis I, Ottesen RA, Hughes ME, Mamet R, Burstein HJ, Edge SB, Gonzalez-Angulo AM, Moy B, Rugo HS, Theriault RL, Weeks JC, Winer EP, Lin NU (2014) Outcomes by tumor subtype and treatment pattern in women with small, node-negative breast cancer: a multi-institutional study. J Clin Oncol 32:2142–2150. doi:10.1200/JCO.2013.53.1608
Joerger M, Thurlimann B, Huober J (2011) Small HER2-positive, node-negative breast cancer: who should receive systemic adjuvant treatment? Ann Oncol 22:17–23. doi:10.1093/annonc/mdq304
Fehrenbacher L, Capra AM, Quesenberry CP Jr, Fulton R, Shiraz P, Habel LA (2014) Distant invasive breast cancer recurrence risk in human epidermal growth factor receptor 2-positive T1a and T1b node-negative localized breast cancer diagnosed from 2000 to 2006: a cohort from an integrated health care delivery system. J Clin Oncol 32:2151–2158. doi:10.1200/JCO.2013.52.0858
Kiess AP, McArthur HL, Mahoney K, Patil S, Morris PG, Ho A, Hudis CA, McCormick B (2012) Adjuvant trastuzumab reduces locoregional recurrence in women who receive breast-conservation therapy for lymph node-negative, human epidermal growth factor receptor 2-positive breast cancer. Cancer 118:1982–1988. doi:10.1002/cncr.26484
Olszewski AJ, Migdady Y, Boolbol SK, Klein P, Boachie-Adjei K, Sakr BJ, Sikov W, Shao T (2013) Effects of adjuvant chemotherapy in HER2-positive or triple-negative pT1ab breast cancers: a multi-institutional retrospective study. Breast Cancer Res Treat 138:215–223. doi:10.1007/s10549-013-2423-3
Rodrigues MJ, Peron J, Frenel JS, Vano YA, Wassermann J, Debled M, Picaud F, Albiges L, Vincent-Salomon A, Cottu PH (2013) Benefit of adjuvant trastuzumab-based chemotherapy in T1ab node-negative HER2-overexpressing breast carcinomas: a multicenter retrospective series. Ann Oncol 24:916–924. doi:10.1093/annonc/mds536
Vici P, Pizzuti L, Natoli C, Moscetti L, Mentuccia L, Vaccaro A, Sergi D, Di Lauro L, Trenta P, Seminara P, Santini D, Iezzi L, Tinari N, Bertolini I, Sini V, Mottolese M, Giannarelli D, Giotta F, Maugeri-Sacca M, Barba M, Marchetti P, Michelotti A, Sperduti I, Gamucci T (2014) Outcomes of HER2-positive early breast cancer patients in the pre-trastuzumab and trastuzumab eras: a real-world multicenter observational analysis. The RETROHER study. Breast Cancer Res Treat 147:599–607. doi:10.1007/s10549-014-3133-1
Tognela A, Beith J, Kiely B, Bastick P, Lynch J, Descallar J, Mok K (2015) Small HER2-positive breast cancer: should size affect adjuvant treatment? Clin Breast Cancer. doi:10.1016/j.clbc.2014.12.012
Gori S, Inno A, Fiorio E, Foglietta J, Ferro A, Gulisano M, Pinotti G, Gubiotti M, Cavazzini MG, Turazza M, Duranti S, De Simone V, Iezzi L, Bisagni G, Spazzapan S, Cavanna L, Saggia C, Bria E, Cretella E, Vici P, Santini D, Fabi A, Garrone O, Frassoldati A, Amaducci L, Saracchini S, Evangelisti L, Barni S, Gamucci T, Mentuccia L, Laudadio L, Zoboli A, Marchetti F, Bogina G, Lunardi G, Boni L (2015) The Promher study: an observational Italian study on adjuvant therapy for HER2-positive, pT1a-b pN0 breast cancer. PLoS One 10:e0136731. doi:10.1371/journal.pone.0136731
Musolino A, Boggiani D, Sikokis A, Rimanti A, Pellegrino B, Vattiato R, Sgargi P, Falcini F, Caminiti C, Michiara M, Leonardi F (2016) Prognostic risk factors for treatment decision in pT1a, b N0M0 HER2-positive breast cancers. Cancer Treat Rev 43:1–7. doi:10.1016/j.ctrv.2015.11.010
O’Sullivan CC, Bradbury I, Campbell C, Spielmann M, Perez EA, Joensuu H, Costantino JP, Delaloge S, Rastogi P, Zardavas D, Ballman KV, Holmes E, de Azambuja E, Piccart-Gebhart M, Zujewski JA, Gelber RD (2015) Efficacy of adjuvant trastuzumab for patients with human epidermal growth factor receptor 2-positive early breast cancer and tumors ≤2 cm: a meta-analysis of the randomized trastuzumab trials. J Clin Oncol. doi:10.1200/JCO.2015.60.8620
Schouten LJ, Hoppener P, van den Brandt PA, Knottnerus JA, Jager JJ (1993) Completeness of cancer registration in Limburg, The Netherlands. Int J Epidemiol 22:369–376
Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, Dowsett M, Fitzgibbons PL, Hanna WM, Langer A, McShane LM, Paik S, Pegram MD, Perez EA, Press MF, Rhodes A, Sturgeon C, Taube SE, Tubbs R, Vance GH, van de Vijver M, Wheeler TM, Hayes DF (2007) American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol 25:118–145. doi:10.1200/JCO.2006.09.2775
van der Heiden-van der Loo M, Schaapveld M, Ho VK, Siesling S, Rutgers EJ, Peeters PH (2013) Outcomes of a population-based series of early breast cancer patients with micrometastases and isolated tumour cells in axillary lymph nodes. Ann Oncol 24:2794–2801. doi:10.1093/annonc/mdt243
Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, Gianni L, Baselga J, Bell R, Jackisch C, Cameron D, Dowsett M, Barrios CH, Steger G, Huang CS, Andersson M, Inbar M, Lichinitser M, Lang I, Nitz U, Iwata H, Thomssen C, Lohrisch C, Suter TM, Ruschoff J, Suto T, Greatorex V, Ward C, Straehle C, McFadden E, Dolci MS, Gelber RD, Herceptin Adjuvant Trial Study Team (2005) Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med 353:1659–1672. doi:10.1056/NEJMoa052306
McArthur HL, Mahoney KM, Morris PG, Patil S, Jacks LM, Howard J, Norton L, Hudis CA (2011) Adjuvant trastuzumab with chemotherapy is effective in women with small, node-negative, HER2-positive breast cancer. Cancer 117:5461–5468. doi:10.1002/cncr.26171
Schroeder MC, Lynch CF, Abu-Hejleh T, Chrischilles EA, Thomas A (2015) Chemotherapy use and surgical treatment by receptor subtype in node-negative T1a and T1b female breast cancers, Iowa SEER registry, 2010- to 2012. Clin Breast Cancer 15:e27–e34. doi:10.1016/j.clbc.2014.07.009
Curigliano G, Viale G, Bagnardi V, Fumagalli L, Locatelli M, Rotmensz N, Ghisini R, Colleoni M, Munzone E, Veronesi P, Zurrida S, Nole F, Goldhirsch A (2009) Clinical relevance of HER2 overexpression/amplification in patients with small tumor size and node-negative breast cancer. J Clin Oncol 27:5693–5699. doi:10.1200/JCO.2009.22.0962
Seferina SC, Lobbezoo DJ, de Boer M, Dercksen MW, van den Berkmortel F, van Kampen RJ, van de Wouw AJ, de Vries B, Joore MA, Peer PG, Voogd AC, Tjan-Heijnen VC (2015) Real-life use and effectiveness of adjuvant trastuzumab in early breast cancer patients: a study of the southeast Netherlands Breast Cancer Consortium. Oncologist 20:856–863. doi:10.1634/theoncologist.2015-0006
Tolaney SM, Barry WT, Dang CT, Yardley DA, Moy B, Marcom PK, Albain KS, Rugo HS, Ellis M, Shapira I, Wolff AC, Carey LA, Overmoyer BA, Partridge AH, Guo H, Hudis CA, Krop IE, Burstein HJ, Winer EP (2015) Adjuvant paclitaxel and trastuzumab for node-negative, HER2-positive breast cancer. N Engl J Med 372:134–141. doi:10.1056/NEJMoa1406281
Gianni L, Pienkowski T, Im YH, Roman L, Tseng LM, Liu MC, Lluch A, Staroslawska E, de la Haba-Rodriguez J, Im SA, Pedrini JL, Poirier B, Morandi P, Semiglazov V, Srimuninnimit V, Bianchi G, Szado T, Ratnayake J, Ross G, Valagussa P (2012) Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol 13:25–32. doi:10.1016/S1470-2045(11)70336-9
Knauer M, Cardoso F, Wesseling J, Bedard PL, Linn SC, Rutgers EJ, van’t Veer LJ (2010) Identification of a low-risk subgroup of HER-2-positive breast cancer by the 70-gene prognosis signature. Br J Cancer 103:1788–1793. doi:10.1038/sj.bjc.6605916
Acknowledgments
The authors thank the Comprehensive Cancer Centre Netherlands for the collection of data in the Netherlands Cancer Registry. In particular, we would like to thank Sabine Siesling, Annemarie Eeltink-Conijn, and Reini Bretveld for their assistance in gathering the clinical data. Furthermore, we thank Statistics Netherlands for the provision of the disease-specific survival data. Lastly, we thank Katarzyna Jozwiak and Erik van Werkhoven of the Netherlands Cancer Institute for their support with the statistical analyses.
Authors’ contributions
Study concept and design: GS. Collection and assembly of data: GS, MvdH, MvR. Data analysis and interpretation: GD, GS, MvdH, MvR, SL. Manuscript writing: GS, MvR. Critical revision of the manuscript for important intellectual content: GD, GS, MvdH, MvR, SL. Final approval of manuscript: GD, GS, MvdH, MvR, SL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests. Relevant financial activities outside the submitted work and other relationships or activities that readers could perceive to have influenced, or that give the appearance of potentially influencing: GS received institutional research support funding from Roche. SL is an advisory board member for Cergentis, Novartis, Roche, and Philips health BV. SL received institutional research support funding from AstraZeneca, Genentech, and Roche.
Additional information
An erratum to this article can be found at http://dx.doi.org/10.1007/s10549-016-3941-6.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
van Ramshorst, M.S., van der Heiden-van der Loo, M., Dackus, G.M.H.E. et al. The effect of trastuzumab-based chemotherapy in small node-negative HER2-positive breast cancer. Breast Cancer Res Treat 158, 361–371 (2016). https://doi.org/10.1007/s10549-016-3878-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-016-3878-9