Abstract
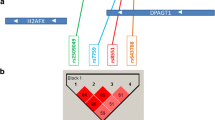
DNA damage recognition and repair is a complex system of genes focused on maintaining genomic stability. Recently, there has been a focus on how breast cancer susceptibility relates to genetic variation in the DNA bypass polymerases pathway. Race-stratified and subtype-specific logistic regression models were used to estimate odds ratios (ORs) and 95 % confidence intervals (CIs) for the association between 22 single-nucleotide polymorphisms (SNPs) in seven bypass polymerase genes and breast cancer risk in the Carolina Breast Cancer Study, a population-based, case–control study (1,972 cases and 1,776 controls). We used SNP-set kernel association test (SKAT) to evaluate the multi-gene, multi-locus (combined) SNP effects within bypass polymerase genes. We found similar ORs for breast cancer with three POLQ SNPs (rs487848 AG/AA vs. GG; OR = 1.31, 95 % CI 1.03–1.68 for Whites and OR = 1.22, 95 % CI 1.00–1.49 for African Americans), (rs532411 CT/TT vs. CC; OR = 1.31, 95 % CI 1.02–1.66 for Whites and OR = 1.22, 95 % CI 1.00–1.48 for African Americans), and (rs3218634 CG/CC vs. GG; OR = 1.29, 95 % CI 1.02–1.65 for Whites). These three SNPs are in high linkage disequilibrium in both races. Tumor subtype analysis showed the same SNPs to be associated with increased risk of Luminal breast cancer. SKAT analysis showed no significant combined SNP effects. These results suggest that variants in the POLQ gene may be associated with the risk of Luminal breast cancer.
Similar content being viewed by others

References
Gieseking S, Bergen K, Di Pasquale F et al (2011) Human DNA polymerase beta mutations allowing efficient abasic site bypass. J Biol Chem 286:4011–4020. doi:10.1074/jbc.M110.176826
Hoeijmakers JH (2001) Genome maintenance mechanisms for preventing cancer. Nature 411:366–374. doi:10.1038/35077232
Lange SS, Takata K, Wood RD (2011) DNA polymerases and cancer. Nat Rev Cancer 11:96–110. doi:10.1038/nrc2998
McCulloch SD, Kunkel TA (2008) The fidelity of DNA synthesis by eukaryotic replicative and translesion synthesis polymerases. Cell Res 18:148–161. doi:10.1038/cr.2008.4
Cleaver JE (2002) Mechanisms by which human cells bypass damaged bases during DNA replication after ultraviolet irradiation. ScientificWorldJournal 2:1296–1305. doi:10.1100/tsw.2002.348
Li X, Heyer WD (2008) Homologous recombination in DNA repair and DNA damage tolerance. Cell Res 18:99–113. doi:10.1038/cr.2008.1
Budzowska M, Kanaar R (2009) Mechanisms of dealing with DNA damage-induced replication problems. Cell Biochem Biophys 53:17–31. doi:10.1007/s12013-008-9039-y
Wood RD, Mitchell M, Lindahl T (2005) Human DNA repair genes, 2005. Mutat Res 577:275–283. doi:10.1016/j.mrfmmm.2005.03.007
Friedberg EC, Gerlach VL (1999) Novel DNA polymerases offer clues to the molecular basis of mutagenesis. Cell 98:413–416
Albertella MR, Lau A, O’Connor MJ (2005) The overexpression of specialized DNA polymerases in cancer. DNA Repair (Amst) 4:583–593. doi:10.1016/j.dnarep.2005.01.005
Choi JH, Pfeifer GP (2005) The role of DNA polymerase eta in UV mutational spectra. DNA Repair (Amst) 4:211–220. doi:10.1016/j.dnarep.2004.09.006
Johnson RE, Kondratick CM, Prakash S et al (1999) hRAD30 mutations in the variant form of xeroderma pigmentosum. Science 285:263–265
Lehmann AR (2000) Replication of UV-damaged DNA: new insights into links between DNA polymerases, mutagenesis and human disease. Gene 253:1–12
Yang J, Chen Z, Liu Y et al (2004) Altered DNA polymerase iota expression in breast cancer cells leads to a reduction in DNA replication fidelity and a higher rate of mutagenesis. Cancer Res 64:5597–5607. doi:10.1158/0008-5472.CAN-04-0603
Wang L, Banerjee S (1995) Mutations in DNA-polymerase-Beta occur in breast, prostate and colorectal tumors. Int J Oncol 6:459–463
Higgins GS, Harris AL, Prevo R et al (2010) Overexpression of POLQ confers a poor prognosis in early breast cancer patients. Oncotarget 1:175–184
Lemee F, Bergoglio V, Fernandez-Vidal A et al (2010) DNA polymerase theta up-regulation is associated with poor survival in breast cancer, perturbs DNA replication, and promotes genetic instability. Proc Natl Acad Sci USA 107:13390–13395. doi:10.1073/pnas.0910759107
Han J, Haiman C, Niu T et al (2009) Genetic variation in DNA repair pathway genes and premenopausal breast cancer risk. Breast Cancer Res Treat 115:613–622. doi:10.1007/s10549-008-0089-z
Monsees GM, Kraft P, Chanock SJ et al (2011) Comprehensive screen of genetic variation in DNA repair pathway genes and postmenopausal breast cancer risk. Breast Cancer Res Treat 125:207–214. doi:10.1007/s10549-010-0947-3
Higgins MJ, Baselga J (2011) Breast cancer in 2010: novel targets and therapies for a personalized approach. Nat Rev Clin Oncol 8:65–66. doi:10.1038/nrclinonc.2010.217
Newman B, Moorman PG, Millikan R et al (1995) The carolina breast cancer study: integrating population-based epidemiology and molecular biology. Breast Cancer Res Treat 35:51–60
Millikan R, Eaton A, Worley K et al (2003) HER2 codon 655 polymorphism and risk of breast cancer in African Americans and Whites. Breast Cancer Res Treat 79:355–364
Aldrich TE, Vann D, Moorman PG et al (1995) Rapid reporting of cancer incidence in a population-based study of breast cancer: one constructive use of a central cancer registry. Breast Cancer Res Treat 35:61–64
Weinberg CR, Sandler DP (1991) Randomized recruitment in case-control studies. Am J Epidemiol 134:421–432
Millikan RC, Player JS, Decotret AR et al (2005) Polymorphisms in DNA repair genes, medical exposure to ionizing radiation, and breast cancer risk. Cancer Epidemiol Biomark Prev 14:2326–2334. doi:10.1158/1055-9965.EPI-05-0186
Nyante SJ (2009) Single nucleotide polymorphisms and the etiology of basal-like and luminal A breast cancer: a pathway-based approach. Ph D dissertation, University of North Carolina at Chapel Hill
Shen R, Fan JB, Campbell D et al (2005) High-throughput SNP genotyping on universal bead arrays. Mutat Res 573:70–82. doi:10.1016/j.mrfmmm.2004.07.022
Barnholtz-Sloan JS, Chakraborty R, Sellers TA et al (2005) Examining population stratification via individual ancestry estimates versus self-reported race. Cancer Epidemiol Biomark Prev 14:1545–1551. doi:10.1158/1055-9965.EPI-04-0832
Nielsen TO, Hsu FD, Jensen K et al (2004) Immunohistochemical and clinical characterization of the basal-like subtype of invasive breast carcinoma. Clin Cancer Res 10:5367–5374. doi:10.1158/1078-0432.CCR-04-0220
Carey LA, Perou CM, Livasy CA et al (2006) Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA 295:2492–2502. doi:10.1001/jama.295.21.2492
Millikan RC, Newman B, Tse CK et al (2008) Epidemiology of basal-like breast cancer. Breast Cancer Res Treat 109:123–139
Huang WY, Newman B, Millikan RC et al (2000) Hormone-related factors and risk of breast cancer in relation to estrogen receptor and progesterone receptor status. Am J Epidemiol 151:703–714
Nyante SJ, Gammon MD, Kaufman JS et al (2011) Common genetic variation in adiponectin, leptin, and leptin receptor and association with breast cancer subtypes. Breast Cancer Res Treat 129:593–606. doi:10.1007/s10549-011-1517-z
Barnholtz-Sloan JS, McEvoy B, Shriver MD et al (2008) Ancestry estimation and correction for population stratification in molecular epidemiologic association studies. Cancer Epidemiol Biomark Prev 17:471–477. doi:10.1158/1055-9965.EPI-07-0491
Pfaff CL, Barnholtz-Sloan J, Wagner JK et al (2004) Information on ancestry from genetic markers. Genet Epidemiol 26:305–315. doi:10.1002/gepi.10319
Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. JSTOR 57:289
Storey JD, Tibshirani R (2003) Statistical methods for identifying differentially expressed genes in DNA microarrays. Methods Mol Biol 224:149–157. doi:10.1385/1-59259-364-X:149
Wu MC, Kraft P, Epstein MP et al (2010) Powerful SNP-set analysis for case-control genome-wide association studies. Am J Hum Genet 86:929–942. doi:10.1016/j.ajhg.2010.05.002
Li H (2012) U-statistics in genetic association studies. Hum Genet. doi:10.1007/s00439-012-1178-y
Schaid DJ (2010) Genomic similarity and Kernel methods I: advancements by building on mathematical and statistical foundations. Hum Hered 70:109–131. doi:10.1159/000312641
Seki M, Wood RD (2008) DNA polymerase theta (POLQ) can extend from mismatches and from bases opposite a (6–4) photoproduct. DNA Repair (Amst) 7:119–127. doi:10.1016/j.dnarep.2007.08.005
Choi JY, Lim S, Kim EJ et al (2010) Translesion synthesis across abasic lesions by human B-family and Y-family DNA polymerases alpha, delta, eta, iota, kappa, and REV1. J Mol Biol 404:34–44. doi:10.1016/j.jmb.2010.09.015
Johnson RE, Haracska L, Prakash S et al (2001) Role of DNA polymerase zeta in the bypass of a (6–4) TT photoproduct. Mol Cell Biol 21:3558–3563. doi:10.1128/MCB.21.10.3558-3563.2001
Wang Z (2001) DNA damage-induced mutagenesis : a novel target for cancer prevention. Mol Interv 1:269–281
Shcherbakova PV, Bebenek K, Kunkel TA (2003) Functions of eukaryotic DNA polymerases. Sci Aging Knowl Environ 2003:RE3
Muzzini DM, Plevani P, Boulton SJ et al (2008) Caenorhabditis elegans POLQ-1 and HEL-308 function in two distinct DNA interstrand cross-link repair pathways. DNA Repair (Amst) 7:941–950. doi:10.1016/j.dnarep.2008.03.021
Hogg M, Seki M, Wood RD et al (2011) Lesion bypass activity of DNA polymerase theta (POLQ) is an intrinsic property of the pol domain and depends on unique sequence inserts. J Mol Biol 405:642–652. doi:10.1016/j.jmb.2010.10.041
Yoon JH, Bhatia G, Prakash S et al (2010) Error-free replicative bypass of thymine glycol by the combined action of DNA polymerases kappa and zeta in human cells. Proc Natl Acad Sci USA 107:14116–14121. doi:10.1073/pnas.1007795107
Prasad R, Longley MJ, Sharief FS et al (2009) Human DNA polymerase theta possesses 5′-dRP lyase activity and functions in single-nucleotide base excision repair in vitro. Nucleic Acids Res 37:1868–1877. doi:10.1093/nar/gkp035
Yoshimura M, Kohzaki M, Nakamura J et al (2006) Vertebrate POLQ and POLbeta cooperate in base excision repair of oxidative DNA damage. Mol Cell 24:115–125. doi:10.1016/j.molcel.2006.07.032
Sjoblom T, Jones S, Wood LD et al (2006) The consensus coding sequences of human breast and colorectal cancers. Science 314:268–274. doi:10.1126/science.1133427
Prat A, Cheang MC, Martin M et al (2013) Prognostic significance of progesterone receptor-positive tumor cells within immunohistochemically defined luminal a breast cancer. J Clin Oncol 31:203–209. doi:10.1200/JCO.2012.43.4134
Cheang MC, Chia SK, Voduc D et al (2009) Ki67 index, HER2 status, and prognosis of patients with luminal B breast cancer. J Natl Cancer Inst 101:736–750. doi:10.1093/jnci/djp082
Kazma R, Babron MC, Gaborieau V et al (2012) Lung cancer and DNA repair genes: multilevel association analysis from the International Lung Cancer Consortium. Carcinogenesis 33:1059–1064. doi:10.1093/carcin/bgs116
McGee SA, Durham DD, Tse CK et al (2013) Determinants of breast cancer treatment delay differ for African American and White women. Cancer Epidemiol Biomark Prev 22:1227–1238. doi:10.1158/1055-9965.EPI-12-1432
Palmer JR, Ambrosone CB, Olshan AF (2014) A collaborative study of the etiology of breast cancer subtypes in African American women: the AMBER consortium. Cancer Causes Control 25:309–319. doi:10.1007/s10552-013-0332-8
Acknowledgments
The authors would like to thank the participants of the Carolina Breast Cancer Study, the UNC BioSpecimen Processing Facility for our DNA extractions, blood processing, storage, and sample disbursement (https://genome.unc.edu/bsp), the UNC Mammalian Genotyping Core for CBCS sample genotyping (http://mgc.unc.edu), and Jessica Tse and Katie O’Brien for their technical assistance and support. Finally, we would like to acknowledge Dr. Robert Millikan, the previous Principal Investigator of the CBCS, for his guidance and inspiration for this paper. This research was funded in part by the University Cancer Research Fund of North Carolina, the National Cancer Institute Specialized Program of Research Excellence (SPORE) in Breast Cancer (NIH/NCI P50-CA58223).
Disclosures
None.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Family, L., Bensen, J.T., Troester, M.A. et al. Single-nucleotide polymorphisms in DNA bypass polymerase genes and association with breast cancer and breast cancer subtypes among African Americans and Whites. Breast Cancer Res Treat 149, 181–190 (2015). https://doi.org/10.1007/s10549-014-3203-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-014-3203-4