Abstract
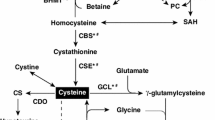
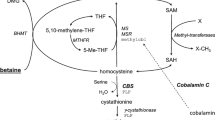
Cystathionine beta synthase (CBS) deficiency is a recessive inborn error of metabolism characterized by elevated serum total homocysteine (tHcy). Betaine supplementation, which can lower tHcy by stimulating homocysteine remethylation to methionine, is often given to CBS deficient patients in combination with other treatments such as methionine restriction and supplemental B-vitamins. However, the effectiveness of betaine supplementation by itself in the treatment of CBS deficiency has not been well explored. Here, we have examined the effect of a betaine supplemented diet on the Tg-I278T Cbs −/− mouse model of CBS deficiency and compared its effectiveness to our previously published data using a methionine restricted diet. Tg-I278T Cbs −/− mice on betaine, from the time of weaning until for 240 days of age, had a 40 % decrease in mean tHcy level and a 137 % increase in serum methionine levels. Betaine-treated Tg-I278T Cbs −/− mice also exhibited increased levels of betaine-dependent homocysteine methyl transferase (BHMT), increased levels of the lipogenic enzyme stearoyl-coenzyme A desaturase (SCD-1), and increased lipid droplet accumulation in the liver. Betaine supplementation largely reversed the hair loss phenotype in Tg-I278T Cbs −/− animals, but was far less effective than methionine restriction in reversing the weight-loss, fat-loss, and osteoporosis phenotypes. Surprisingly, betaine supplementation had several negative effects in control Tg-I278T Cbs +/− mice including decreased weight gain, lean mass, and bone mineral density. Our findings indicate that while betaine supplementation does have some beneficial effects, it is not as effective as methionine restriction for reversing the phenotypes associated with severe CBS deficiency in mice.





Similar content being viewed by others
References
Barber GW, Spaeth GL (1969) The successful treatment of homocystinuria with pyridoxine. J Pediatr 75:463–478
Brenton DP, Dow CJ, James JI, Hay RL, Wynne-Davies R (1972) Homocystinuria and Marfan’s syndrome. A comparison. J Bone Joint Surg (Br) 54:277–298
Chen X, Wang L, Fazlieva R, Kruger WD (2006) Contrasting behaviors of mutant cystathionine beta-synthase enzymes associated with pyridoxine response. Hum Mutat 27:474–482
Cohen P, Miyazaki M, Socci ND et al (2002) Role for stearoyl-CoA desaturase-1 in leptin-mediated weight loss. Science 297:240–243
Dayal S, Chauhan AK, Jensen M et al (2012) Paradoxical absence of a prothrombotic phenotype in a mouse model of severe hyperhomocysteinemia. Blood 119:3176–3183
Devlin AM, Hajipour L, Gholkar A, Fernandes H, Ramesh V, Morris AA (2004) Cerebral edema associated with betaine treatment in classical homocystinuria. J Pediatr 144:545–548
Gupta S, Kruger WD (2011) Cystathionine beta-synthase deficiency causes fat loss in mice. PLoS One 6, e27598
Gupta S, Kühnisch J, Mustafa A et al (2009) Mouse models of cystathionine β-synthase deficiency reveal significant threshold effects of hyperhomocysteinemia. FASEB J 23:883–893
Gupta S, Melnyk SB, Kruger WD (2014) Cystathionine beta-synthase-deficient mice thrive on a low-methionine diet. FASEB J 28:781–790
Heinemann FS, Ozols J (1998) Degradation of stearoyl-coenzyme A desaturase: endoproteolytic cleavage by an integral membrane protease. Mol Biol Cell 9:3445–3453
Komrower GM, Lambert AM, Cusworth DC, Westall RG (1966) Dietary treatment of homocystinuria. Arch Dis Child 41:666–671
Maclean KN, Jiang H, Greiner LS, Allen RH, Stabler SP (2012) Long-term betaine therapy in a murine model of cystathionine beta-synthase deficient homocystinuria: decreased efficacy over time reveals a significant threshold effect between elevated homocysteine and thrombotic risk. Mol Genet Metab 105:395–403
Maclean KN, Sikora J, Kozich V, et al (2010) A novel transgenic mouse model of CBS-deficient homocystinuria does not incur hepatic steatosis or fibrosis and exhibits a hypercoagulative phenotype that is ameliorated by betaine treatment. Mol Genet Metab
Mudd SH, Levy HL, Kraus JP (2001) Disorders in transsulfuration. In: Scriver CR, Beaudet A, Sly W, Valle D (eds) The metabolic basis of inherited disease. McGraw-Hill, New York, pp 2007–2056
Paton CM, Ntambi JM (2009) Biochemical and physiological function of stearoyl-CoA desaturase. Am J Phys Endo and Metab 297:E28–E37
Perry TL, Dunn HG, Hansen S, MacDougall L, Warrington PD (1966) Early diagnosis and treatment of homocystinuria. Pediatrics 37:502–505
Poloni S, Leistner-Segal S, Bandeira IC et al (2014) Body composition in patients with classical homocystinuria: body mass relates to homocysteine and choline metabolism. Gene 546:443–447
Reagan-Shaw S, Nihal M, Ahmad N (2008) Dose translation from animal to human studies revisited. FASEB J 22:659–661
Robert K, Maurin N, Ledru A, Delabar J, Janel N (2004) Hyperkeratosis in cystathionine beta synthase-deficient mice: an animal model of hyperhomocysteinemia. Anat Rec A: Discov Mol Cell Evol Biol 280:1072–1076
Sakamoto A, Sakura N (2003) Limited effectiveness of betaine therapy for cystathionine beta synthase deficiency. Pediatr Int 45:333–338
Sardharwalla IB, Jackson SH, Hawke HD, Sass-Kortsak A (1968) Homocystinuria: a study with low-methionine diet in three patients. Can Med Assoc J 99:731–740
Wang L, Chen X, Tang B, Hua X, Klein-Szanto A, Kruger WD (2005) Expression of mutant human cystathionine beta-synthase rescues neonatal lethality but not homocystinuria in a mouse model. Hum Mol Genet 14:2201–2208
Wang L, Jhee KH, Hua X, DiBello PM, Jacobsen DW, Kruger WD (2004) Modulation of cystathionine beta-synthase level regulates total serum homocysteine in mice. Circ Res 94:1318–1324
Wilcken DE, Wilcken B, Dudman NP, Tyrrell PA (1983) Homocystinuria—the effects of betaine in the treatment of patients not responsive to pyridoxine. N Engl J Med 309:448–453
Yaghmai R, Kashani AH, Geraghty MT et al (2002) Progressive cerebral edema associated with high methionine levels and betaine therapy in a patient with cystathionine beta-synthase (CBS) deficiency. Am J Med Genet 108:57–63
Yap S, Boers GH, Wilcken B et al (2001) Vascular outcome in patients with homocystinuria due to cystathionine beta-synthase deficiency treated chronically: a multicenter observational study. Arterioscler Thromb Vasc Biol 21:2080–2085
Yap S, Naughten E (1998) Homocystinuria due to cystathionine beta-synthase deficiency in Ireland: 25 years’ experience of a newborn screened and treated population with reference to clinical outcome and biochemical control [In Process Citation]. J Inherit Metab Dis 21:738–747
Acknowledgments
This work was supported by grants from the Hempling Foundation for Homocystinuria Research, the NIH (CA06927 and R01GM098772), and an appropriation from the Commonwealth of Pennsylvania. We thank the Genomics and Laboratory Animal Facilities of Fox Chase Cancer Center for their assistance. We also thank Dr. Michael Tordoff and his laboratory for help using the PIXIMus II DEXA. This equipment was provided by funds awarded to the Monell Chemical Senses Center under the grant from the Pennsylvania Department of Health. The department specifically disclaims responsibility for any analyses, interpretations, or conclusions of the study.
Conflict of interest
None.
Animal Rights
All institutional and national guidelines for the care and use of laboratory animals were followed.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by: Matthias Baumgartner
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig. 1
Liver pathology of RD and BSD animals. For each animal on the study, liver pathology was assessed by H and E staining. Slides were scored either positive or negative for the presence of fat droplets in a blinded fashion. Representative H and E’s are shown. The table below shows the number of positive and total animals in each group. (PDF 61 kb)
Supplementary Fig. 2
BHMT activity. Liver extracts from mice with the indicated genotype and on the indicated diets were assessed for BHMT activity as described in Methods. Activity is expressed as a percentage of the control group (Cbs+/− mice on RD). N = 6 for each group. Bars show 95 % CI (PDF 120 kb)
Rights and permissions
About this article
Cite this article
Gupta, S., Wang, L. & Kruger, W.D. Betaine supplementation is less effective than methionine restriction in correcting phenotypes of CBS deficient mice. J Inherit Metab Dis 39, 39–46 (2016). https://doi.org/10.1007/s10545-015-9883-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10545-015-9883-z