Abstract
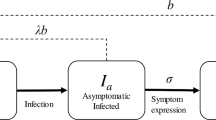
Pine wilt disease is caused by the pinewood nematode Bursaphelenchus xylophilus, which is vectored by the Japanese pine sawyer beetle Monochamus alternatus. Due to their mutualistic relationship, according to which the nematode weakens and makes trees available for beetle reproduction and the beetle in turn carries and transmits the nematode to healthy pine trees, this disease has resulted in severe damage to pine trees in Japan in recent decades. Previous studies have worked on modeling of population dynamics of the vector beetle and the pine tree to explore spatial expansion of the disease using an integro-difference equation with a dispersal kernel that describes beetle mobility over space. In this paper, I revisit these previous models but retaining individuality: by considering mechanistic interactions at the individual level it is shown that the Allee effect, an increasing per-capita growth rate as population abundance increases, can arise in the beetle dynamics because of the necessity for beetles to contact pine trees at least twice to reproduce successfully. The incubation period after which a tree contacted by a first beetle becomes ready for beetle oviposition by later beetles is crucial for the emergence of this Allee effect. It is also shown, however, that the strength of this Allee effect depends strongly on biological mechanistic properties, especially on beetle mobility. Realistic individual-based modeling highlights the importance of how spatial scales are dealt with in mathematical models. The link between mechanistic individual-based modeling and conventional analytical approaches is also discussed.






Similar content being viewed by others
References
Beckenbach K, Smith MJ, Webster JM (1992) Taxonomic affinities and intra- and interspecitic variation in Bursaphelenchus spp. as determined by polymerase chain reaction. J Nematol 24:140–147
Berec L, Boukal DS (2004) Implications of mate search, mate choice and divorce rate for population dynamics of sexually reproducing species. Oikos 104:122–142. doi:10.1111/j.0030-1299.2004.12753.x
Boukal DS, Berec L (2002) Single-species models of the Allee effect: extinction boundaries, sex ratios and mate encounters. J Theor Biol 218:375–394. doi:10.1006/jtbi.2002.3084
Clark JS (1998) Why trees migrate so fast: confronting theory with dispersal biology and the paleorecord. Am Nat 152:204–224. doi:10.1086/286162
Dennis B (1989) Allee effects: population growth, critical density, and the chance of extinction. Nat Res Model 3:481–538
Dennis B (2002) Allee effects in stochastic populations. Oikos 96:389–401. doi:10.1034/j.1600-0706.2002.960301.x
Dieckmann U, Law R, Metz JAJ (eds) (2000) The geometry of ecological interactions—simplifying spatial complexity. Cambridge University Press, Cambridge
Durrett R, Levin S (1994) The importance of being discrete (and spatial). Theor Popul Biol 46:363–394. doi:10.1006/tpbi.1994.1032
Fujioka H (1993) A report on the habitat of Monochamus alternatus Hope in Akita prefecture. Bull Akita For Tech Cent 2:40–56 (in Japanese with English summary)
Futai K, Furuno T (1979) The variety of resistances among pine-species to pine wood nematode, Bursaphelenchus lignicolus. Bull Kyoto Univ For 51:23–36
Hastings A, Cuddington K, Davies KF, Dugaw CJ, Elmendort S, Freestone A, Harrison S, Holland M, Lambrinos J, Malvadkar U, Melbourne BA, Moore K, Taylor C, Thomson D (2005) The spatial spread of invasions: new developments in theory and evidence. Ecol Lett 8:91–101. doi:10.1111/j.1461-0248.2004.00687.x
Ikeda T, Oda K (1980) The occurrence of attractiveness for Monochamus alternatus Hope (Coleoptera: Cerambycidae) in nematode-infected pine trees. J Jpn For Soc 62:432–434
Kishi Y (1995) The pine wood nematode and the Japanese pine sawyer. Thomas Company, Tokyo
Kiyohara T, Tokushige Y (1971) Inoculation experiments of a nematode, Bursaphelenchus sp., onto pine trees. J Jpn For Soc 53:210–218 (in Japanese with English summary)
Kot M, Schaffer WM (1986) Discrete-time growth-dispersal models. Math Biosci 80:109–136. doi:10.1016/0025-5564(86)90069-6
Kot M, Lewis MA, van den Driessche P (1996) Dispersal data and the spread of invading organisms. Ecology 77:2027–2042. doi:10.2307/2265698
Kot M, Medlock J, Reluga T, Walton DB (2004) Stochasticity, invasions and branching random walks. Theor Popul Biol 66:175–184. doi:10.1016/j.tpb.2004.05.005
Law R, Murrell DJ, Dieckmann U (2003) Population growth in space and time: spatial logistic equations. Ecology 84(1):252–262. doi:10.1890/0012-9658(2003)084[0252:PGISAT]2.0.CO;2
Lewis MA (1997) Variability, patchness, and jump dispersal in the spread of an invading population. In: Tilman D, Karieva P (eds) Spatial ecology: the role of space in population dynamics and interspecific interactions. Princeton University Press, Princeton, pp 46–69
Malek NB, Appleby JE (1984) Epidemiology of pine wilt in Illinois. Plant Dis 68:180–186. doi:10.1094/PD-69-180
Mamiya Y (1987) Origin of the pine wood nematode and its distribution outside the United States. In: Wingfield MJ (ed) Pathogenecity of the pine wood nematode. American Phytopathological Society Press, Saint Paul, Minnesota, pp 59–65
Mamiya Y (1988) History of pine wilt disease in Japan. J Nematol 20:219–226
Mamiya Y, Enda N (1972) Transmission of Bursaphelenchus lignicolus (Nematoda: Aphelenchoididae) by Monochamus alternatus (Coleoptera: Cerambycidae). Nematologica 18:159–162
Mamiya Y, Kiyohara T (1972) Description of Bursaphelenchus lignicolus (Nematoda: Aphelenchoididae) by Monochamus alternatus (Colepotera: Cerambycidae). Nematologica 18:120–1124
Morimoto K, Iwasaki A (1972) Role of Monochamus alternatus (Coleoptera: Cerambycidae) as a vector of Bursaphelenchus lignicolus (Nematoda: Aphelenchoididae). J Jpn For Soc 54:177–182 (in Japanese with English summary)
Neubert MG, Kot M, Lewis MA (1995) Dispersal and pattern formation in a discrete-time predator-prey model. Theor Popul Biol 48:7–43. doi:10.1006/tpbi.1995.1020
Neubert MG, Kot M, Lewis MA (2000) Invasion speeds in fluctuating environments. Proc R Soc B 267:1603–1610 (Errata: 267:2568–2569)
Rutherford TA, Webster JM (1987) Distribution of pine wilt disease with respect to temperature in North America, Japan. Can J For Res 17:1050–1059. doi:10.1139/x87-161
Shigesada N, Kawasaki K (1997) Biological invasions: theory and practice. Oxford series in ecology and evolution. Oxford University Press, Oxford
Shigesada N, Kawasaki K (2002) Invasion and the range expansion of species: effects of long-distance dispersal. In: Bullock JM, Kenward RE, Hails RS (eds) Dispersal ecology: the 42nd symposium of the British ecological society, Blackwell, pp 350–373
Snyder RE (2003) How demographic stochasticity can slow biological invasions. Ecology 84(5):1333–1339. doi:10.1890/0012-9658(2003)084[1333:HDSCSB]2.0.CO;2
Stephens PA, Sutherland WJ (1999) Consequences of the Allee effect for behavior, ecology and conservation. Trends Ecol Evol 14:401–405. doi:10.1016/S0169-5347(99)01684-5
Takasu F, Yamamoto N, Kawasaki K, Togashi K, Shigesada N (2000) Modeling the expansion of an introduced tree disease. Biol Invasions 2:141–150. doi:10.1023/A:1010048725497
Togashi K, Shigesada N (2006) Spread of the pinewood nematode vectored by the Japanese pine sawyer: modeling and analytical approaches. Popul Ecol 48:271–283. doi:10.1007/s10144-006-0011-7
Wang MH, Kot M, Neubert MG (2002) Integrodifference equations, Allee effects, and invasions. J Math Biol 44:150–168. doi:10.1007/s002850100116
Wingfield MJ, Blanchette RA, Nicholls TH (1984) Is the pine wood nematode an important pathogen in the United States? J For 82:232–235
Yoshimura A, Kawasaki K, Taksau F, Togashi K, Futai K, Shigesada N (1999) Modeling the spread of pine wilt disease caused by nematodes with pine sawyers as vector. Ecology 80(5):1691–1702
Acknowledgments
I thank K. Kawasaki and N. Shigesada for valuable comments and discussion on an earlier draft. I also thank two anonymous reviewers for valuable comments that improved this manuscript. This work was supported in part by KAKENHI, JSPS Grant-in-Aid for Scientific Research (C) 18570020.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Takasu, F. Individual-based modeling of the spread of pine wilt disease: vector beetle dispersal and the Allee effect. Popul Ecol 51, 399–409 (2009). https://doi.org/10.1007/s10144-009-0145-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10144-009-0145-5