Abstract
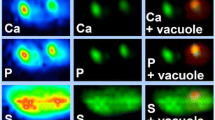
Synchrotron radiation induced X-ray emission (SRIXE) spectroscopy was used to map the cellular uptake of the organoselenium-based antioxidant drug ebselen using differentiated ND15 cells as a neuronal model. The cellular SRIXE spectra, acquired using a hard X-ray microprobe beam (12.8-keV), showed a large enhancement of fluorescence at the Kα line for Se (11.2-keV) following treatment with ebselen (10 μM) at time periods from 60 to 240 min. Drug uptake was quantified and ebselen was shown to induce time-dependent changes in cellular elemental content that were characteristic of oxidative stress with the efflux of K, Cl, and Ca species. The SRIXE cellular Se distribution map revealed that ebselen was predominantly localized to a discreet region of the cell which, by comparison with the K and P elemental maps, is postulated to correspond to the endoplasmic reticulum. On the basis of these findings, it is hypothesized that a major outcome of ebselen redox catalysis is the induction of cellular stress. A mechanism of action of ebselen is proposed that involves the cell responding to drug-induced stress by increasing the expression of antioxidant genes. This hypothesis is supported by the observation that ebselen also regulated the homeostasis of the transition metals Mn, Cu, Fe, and Zn, with increases in transition metal uptake paralleling known induction times for the expression of antioxidant metalloenzymes.






Similar content being viewed by others
Abbreviations
- ARE:
-
Antioxidant response element
- ER:
-
Endoplasmic reticulum
- GPx:
-
Glutathione peroxidase
- GSH:
-
Reduced glutathione
- GSSG:
-
Oxidized glutathione
- ROS:
-
Reactive oxygen species
- SOD:
-
Superoxide dismutase
- SRIXE:
-
Synchrotron-radiation-induced X-ray emission
References
Yamaguchi T, Sano K, Takakura K, Saito I, Shinohara Y, Asano T, Yasuhara H (1998) Stroke 29:12–17
Mugesh G, du Mont WW, Sies H (2001) Chem Rev 101:2125–2179
Giles NM, Gutowski NJ, Giles GI, Jacob C (2003) FEBS Lett 535:179–182
Giles NM, Giles GI, Holley JE, Gutowski NJ, Jacob C (2003) Biochem Pharmacol 66:2021–2028
Giles GI, Fry FH, Tasker KM, Holme AL, Peers C, Green KN, Klotz LO, Sies H, Jacob C (2003) Org Biomol Chem 1:4317–4322
Giles GI, Giles NM, Collins CA, Holt K, Fry FH, Lowden PA, Gutowski NJ, Jacob C (2003) Chem Commun 2030–2031
Fry FH, Holme AL, Giles NM, Giles GI, Collins C, Holt K, Pariagh S, Gelbrich T, Hursthouse MB, Gutowski NJ, Jacob C (2005) Org Biomol Chem 3:2579–2587
Giles GI, Tasker KM, Johnson RJ, Jacob C, Peers C, Green KN (2001) Chem Commun 2490–2491
Yamagata K, Ichinose S, Miyashita A, Tagami M (2008) Neuroscience 153:428–435
Lapchak PA, Zivin JA (2003) Stroke 34:2013–2018
Salom JB, Perez-Asensio FJ, Burguete MC, Marin N, Pitarch C, Torregrosa G, Romero FJ, Alborch E (2004) Eur J Pharmacol 495:55–62
Muller A, Cadenas E, Graf P, Sies H (1984) Biochem Pharmacol 33:3235–3239
Arthur JR (2000) Cell Mol Life Sci 57:1825–1835
Zhao R, Holmgren A (2002) J Biol Chem 277:39456–39462
Zhao R, Masayasu H, Holmgren A (2002) Proc Natl Acad Sci USA 99:8579–8584
Haenen GR, De Rooij BM, Vermeulen NP, Bast A (1990) Mol Pharmacol 37:412–422
Jacob C, Maret W, Vallee BL (1998) Biochem Biophys Res Commun 248:569–573
Xia R, Ganther HE, Egge A, Abramson JJ (2004) Biochem Pharmacol 67:2071–2079
Blessing H, Kraus S, Heindl P, Bal W, Hartwig A (2004) Eur J Biochem 271:3190–3199
Larabee JL, Hocker JR, Hanas JS (2009) J Inorg Biochem 103:419–426
Sarma BK, Mugesh G (2005) J Am Chem Soc 127:11477–11485
Sakurai T, Kanayama M, Shibata T, Itoh K, Kobayashi A, Yamamoto M, Uchida K (2006) Chem Res Toxicol 19:1196–1204
Walther M, Holzhutter HG, Kuban RJ, Wiesner R, Rathmann J, Kuhn H (1999) Mol Pharmacol 56:196–203
Nakamura Y, Feng Q, Kumagai T, Torikai K, Ohigashi H, Osawa T, Noguchi N, Niki E, Uchida K (2002) J Biol Chem 277:2687–2694
Zembowicz A, Hatchett RJ, Radziszewski W, Gryglewski RJ (1993) J Pharmacol Exp Ther 267:1112–1118
Cotgreave IA, Duddy SK, Kass GE, Thompson D, Moldeus P (1989) Biochem Pharmacol 38:649–656
Tamasi V, Jeffries JM, Arteel GE, Falkner KC (2004) Arch Biochem Biophys 431:161–168
Duvvuri M, Konkar S, Funk RS, Krise JM, Krise JP (2005) Biochemistry 44:15743–15749
Torchilin VP (2006) Annu Rev Biomed Eng 8:343–375
Spasojevic I, Chen Y, Noel TJ, Yu Y, Cole MP, Zhang L, Zhao Y, St Clair DK, Batinic-Haberle I (2007) Free Radic Biol Med 42:1193–1200
Kehr S, Malinouski M, Finney L, Vogt S, Labunskyy VM, Kasaikina MV, Carlson BA, Zhou Y, Hatfield DL, Gladyshev VN (2009) J Mol Biol 389:808–818
Aitken JB, Carter EA, Eastgate H, Hackett MJ, Harris HH, Levina A, Lee YC, Chen CI, Lai B, Vogt S, Lay PA (2010) Radiat Phys Chem 79:176–184
Aitken JB, Levina A, Lay PA (2011) Curr Top Med Chem 11:553–571
Carter EA, Rayner BS, McLeod AI, Wu LE, Marshall CP, Levina A, Aitken JB, Witting PK, Lai B, Cai Z, Vogt S, Lee YC, Chen CI, Tobin MJ, Harris HH, Lay PA (2010) Mol Biosyst 6:1316–1322
Yang L, McRae R, Henary MM, Patel R, Lai B, Vogt S, Fahrni CJ (2005) Proc Natl Acad Sci USA 102:11179–11184
Duong TTH, Witting PK, Antao ST, Parry SN, Kennerson M, Lai B, Vogt S, Lay PA, Harris HH (2009) J Neurochem 108:1143–1154
Witting PK, Harris HH, Rayner BS, Aitken JB, Dillon CT, Stocker R, Lai B, Cai Z, Lay PA (2006) Biochemistry 45:12500–12509
Harris HH, Levina A, Dillon CT, Mulyani I, Lai B, Cai Z, Lay PA (2005) J Biol Inorg Chem 10:105–118
Cai Z, Lai B, Yun W, Ilinski P, Legnini D, Maser J, Rodrigues W (2000) Am Inst Phys Proc 507:472–477
Vogt S (2003) J Phys Paris IV 104:635–638
Van Espen P (2002) In: Van Grieken R, Markowicz A (eds) Handbook of X-ray spectrometry: revised and expanded. Dekker, New York, p 29
Waern JB, Harris HH, Lai B, Cai Z, Harding MM, Dillon CT (2005) J Biol Inorg Chem 10:443–452
Dillon CT, Lay PA, Kennedy BJ, Stampfl AP, Cai Z, Ilinski P, Rodrigues W, Legnini DG, Lai B, Maser J (2002) J Biol Inorg Chem 7:640–645
Yadav S, Zajac E, Singhal SS, Awasthi S (2007) Cancer Metastasis Rev 26:59–69
Rayner B, Harris H, Carter E, Vogt S, Cai Z, Lai B, Chin C, Lee Y, Lay P, Witting P (2006) Atherosclerosis 7:S587
Ferreri-Jacobia M, Mak DO, Foskett JK (2005) J Biol Chem 280:3824–3831
White JK, Stewart A, Popoff JF, Wilson S, Blackwell JM (2004) Biochem J 382:811–819
Kruusma J, Benham AM, Williams JA, Kataky R (2006) Analyst 131:459–473
Jung CH, Washburn MP, Wells WW (2002) Biochem Biophys Res Commun 291:550–553
Ross MF, Prime TA, Abakumova I, James AM, Porteous CM, Smith RA, Murphy MP (2008) Biochem J 411:633–645
Gorlach A, Klappa P, Kietzmann T (2006) Antioxid Redox Signal 8:1391–1418
Liu Y, Kern JT, Walker JR, Johnson JA, Schultz PG, Luesch H (2007) Proc Natl Acad Sci USA 104:5205–5210
Park EY, Rho HM (2002) Mol Cell Biochem 240:47–55
Na HK, Kim EH, Jung JH, Lee HH, Hyun JW, Surh YJ (2008) Arch Biochem Biophys 476:171–177
Coleman JE (1992) Annu Rev Biochem 61:897–946
Iwasaki K, Hailemariam K, Tsuji Y (2007) J Biol Chem 282:22335–22343
Purdom-Dickinson SE, Lin Y, Dedek M, Morrissy S, Johnson J, Chen QM (2007) J Mol Cell Cardiol 42:159–176
Schmidt M, Zahn S, Carella M, Ohlenschlager O, Gorlach M, Kothe E, Weston J (2008) Chembiochem 9:2135–2146
Acknowledgments
We are grateful for financial support provided by a University of Sydney Postdoctoral Fellowship (G.I.G), Australian Research Council (ARC) Discovery grants (P.A.L., H.H.H., and P.K.W.), including an ARC Australian Professorial Fellowship (to P.A.L), an ARC QEII Fellowship (to H.H.H.), and an ARC Australian Research Fellowship (to P.K.W.), a National Heart Foundation Grant-in-Aid (to P.K.W), and an Australian Synchrotron Research Program (ASRP) grant for access to the Advanced Photon Source facilities. The ASRP was funded by the Commonwealth of Australia under the Major National Research Facilities Program. The use of Advanced Photon Source facilities was supported by the US Department of Energy, Basic Energy Sciences, Office of Science, under contract number W-31-109-Eng-38.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Aitken, J.B., Lay, P.A., Duong, T.T.H. et al. Synchrotron radiation induced X-ray emission studies of the antioxidant mechanism of the organoselenium drug ebselen. J Biol Inorg Chem 17, 589–598 (2012). https://doi.org/10.1007/s00775-012-0879-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00775-012-0879-y