Abstract
Background
Most studies evaluating visceral sensation measure visceromotor response (VMR) to colorectal distention (CRD). However, CRD itself induces visceral sensitization, and little is known about the detailed characteristics of this response. The present study tried to clarify this question.
Methods
VMR was determined by measuring abdominal muscle contractions as a response to CRD in rats. The CRD set consisted of two isobaric distentions (60 mmHg for 10 min twice, with a 30-min rest), and the CRD set was performed on two separate days, i.e., days 1 and 3, 8.
Results
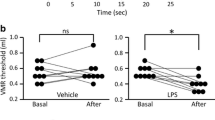
On day 1, VMR to the second CRD was increased as compared with that to the first CRD, which is the acute sensitization. VMR to the first CRD on day 3 returned to the same level as that to the first CRD on day 1, and total VMR, i.e., the whole response to the CRD set, was not different between day 1 and day 3. However, total VMR was significantly increased on day 8 as compared with that on day 1, suggesting CRD induced the delayed sensitization. Intraperitoneally administered astressin (200 µg/kg), a corticotropin-releasing factor receptor antagonist, at the end of the first CRD blocked the acute sensitization, but anakinra (20 mg/kg, intraperitoneally), an interleukin-1 receptor antagonist, did not modify it. Astressin (200 µg/kg, twice before CRD on day 8) did not alter the delayed sensitization, but anakinra (20 mg/kg, twice) abolished it.
Conclusions
CRD induced both acute sensitization and delayed sensitization, which were mediated through peripheral corticotropin-releasing factor and interleukin-1 pathways, respectively.







Similar content being viewed by others
References
Lee YJ, Park KS. Irritable bowel syndrome: emerging paradigm in pathophysiology. World J Gastroenterol. 2014;20:2456–69.
Taché Y, Martínez V, Wang L, et al. CRF1 receptor signaling pathways are involved in stress-related alterations of colonic function and viscerosensitivity: implications for irritable bowel syndrome. Br J Pharmacol. 2004;141:1321–30.
Tanaka Y, Kanazawa M, Fukudo S, et al. Biopsychosocial model of irritable bowel syndrome. J Neurogastroenterol Motil. 2011;17:131–9.
Owens MJ, Nemeroff CB. Physiology and pharmacology of corticotropin-releasing factor. Pharmacol Rev. 1991;43:425–73.
Million M, Zhao JF, Luckey A, et al. The newly developed CRF1-receptor antagonists, NGD 98-2 and NGD 9002, suppress acute stress-induced stimulation of colonic motor function and visceral hypersensitivity in rats. PLoS One. 2013;8:e73749.
Nozu T, Takakusaki K, Okumura T. A balance theory of peripheral corticotropin-releasing factor receptor type 1 and type 2 signaling to induce colonic contractions and visceral hyperalgesia in rats. Endocrinology. 2014;155:4655–64.
Saito-Nakaya K, Hasegawa R, Nagura Y, et al. Corticotropin-releasing hormone receptor 1 antagonist blocks colonic hypersensitivity induced by a combination of inflammation and repetitive colorectal distension. Neurogastroenterol Motil. 2008;20:1147–56.
Ait-Belgnaoui A, Bradesi S, Fioramonti J, et al. Acute stress-induced hypersensitivity to colonic distension depends upon increase in paracellular permeability: role of myosin light chain kinase. Pain. 2005;113:141–7.
Schwetz I, Bradesi S, McRoberts JA, et al. Delayed stress-induced colonic hypersensitivity in male Wistar rats: role of neurokinin-1 and corticotropin-releasing factor-1 receptors. Am J Physiol Gastrointest Liver Physiol. 2004;286:G683–91.
Million M, Maillot C, Adelson DA, et al. Peripheral injection of sauvagine prevents repeated colorectal distension-induced visceral pain in female rats. Peptides. 2005;26:1188–95.
Santos J, Saunders PR, Hanssen NP, et al. Corticotropin-releasing hormone mimics stress-induced colonic epithelial pathophysiology in the rat. Am J Physiol Gastrointest Liver Physiol. 1999;277:G391–9.
Larauche M, Gourcerol G, Wang L, et al. Cortagine, a CRF1 agonist, induces stresslike alterations of colonic function and visceral hypersensitivity in rodents primarily through peripheral pathways. Am J Physiol Gastrointest Liver Physiol. 2009;297:G215–27.
Larauche M, Kiank C, Taché Y. Corticotropin releasing factor signaling in colon and ileum: regulation by stress and pathophysiological implications. J Physiol Pharmacol. 2009;60(Suppl 7):33–46.
Liebregts T, Adam B, Bredack C, et al. Immune activation in patients with irritable bowel syndrome. Gastroenterology. 2007;132:913–20.
Coelho AM, Fioramonti J, Bueno L. Systemic lipopolysaccharide influences rectal sensitivity in rats: role of mast cells, cytokines, and vagus nerve. Am J Physiol Gastrointest Liver Physiol. 2000;279:G781–90.
Girard S, Tremblay L, Lepage M, et al. IL-1 receptor antagonist protects against placental and neurodevelopmental defects induced by maternal inflammation. J Immunol. 2010;184:3997–4005.
Tsuchiya Y, Nozu T, Kumei S, et al. IL-1 receptor antagonist blocks the lipopolysaccharide-induced inhibition of gastric motility in freely moving conscious rats. Dig Dis Sci. 2012;57:2555–61.
Gaudreau GA, Plourde V. Involvement of N-methyl-d-aspartate (NMDA) receptors in a rat model of visceral hypersensitivity. Behav Brain Res. 2004;150:185–9.
Gage GJ, Kipke DR, Shain W. Whole animal perfusion fixation for rodents. J Vis Exp. 2012;(65):e3564. doi:10.3791/3564.
Bradesi S, Schwetz I, Ennes HS, et al. Repeated exposure to water avoidance stress in rats: a new model for sustained visceral hyperalgesia. Am J Physiol Gastrointest Liver Physiol. 2005;289:G42–53.
Larauche M, Mulak A, Kim YS, et al. Visceral analgesia induced by acute and repeated water avoidance stress in rats: sex difference in opioid involvement. Neurogastroenterol Motil. 2012;24:1031-e547.
Stengel A, Taché Y. Neuroendocrine control of the gut during stress: corticotropin-releasing factor signaling pathways in the spotlight. Annu Rev Physiol. 2009;71:219–39.
Million M, Wang L, Wang Y, et al. CRF2 receptor activation prevents colorectal distension induced visceral pain and spinal ERK1/2 phosphorylation in rats. Gut. 2006;55:172–81.
Wu SV, Yuan PQ, Lai J, et al. Activation of Type 1 CRH receptor isoforms induces serotonin release from human carcinoid BON-1N cells: an enterochromaffin cell model. Endocrinology. 2011;152:126–37.
Overman EL, Rivier JE, Moeser AJ. CRF induces intestinal epithelial barrier injury via the release of mast cell proteases and TNF-α. PLoS One. 2012;7:e39935.
Chaloner A, Greenwood-Van Meerveld B. Genetic diversity contributes to abnormalities in pain behaviors between young and old rats. Age (Dordr). 2013;35:1–10.
Zhai QZ, Traub RJ. The NMDA receptor antagonist MK-801 attenuates c-Fos expression in the lumbosacral spinal cord following repetitive noxious and non-noxious colorectal distention. Pain. 1999;83:321–9.
Vlachakis IK, Pitoulias GA, Kontopoulou KE, et al. Semapimod a new pretreatment modality of acute intestinal ischemia-reperfusion syndrome: experimental study in rabbits. Int Angiol. 2011;30:35–42.
Schultzberg M, Svenson SB, Unden A, et al. Interleukin-1-like immunoreactivity in peripheral tissues. J Neurosci Res. 1987;18:184–9.
Fu LW, Longhurst JC. Interleukin-1β sensitizes abdominal visceral afferents of cats to ischaemia and histamine. J Physiol. 1999;521(1):249–60.
Greenhalgh AD, Galea J, Denes A, et al. Rapid brain penetration of interleukin-1 receptor antagonist in rat cerebral ischaemia: pharmacokinetics, distribution, protection. Br J Pharmacol. 2010;160:153–9.
Galea J, Ogungbenro K, Hulme S, et al. Intravenous anakinra can achieve experimentally effective concentrations in the central nervous system within a therapeutic time window: results of a dose-ranging study. J Cereb Blood Flow Metab. 2011;31:439–47.
Coelho A, Fioramonti J, Bueno L. Brain interleukin-1β and tumor necrosis factor-α are involved in lipopolysaccharide-induced delayed rectal allodynia in awake rats. Brain Res Bull. 2000;52:223–8.
Sloots CE, Felt-Bersma RJ, Cuesta MA, et al. Rectal visceral sensitivity in healthy volunteers: influences of gender, age and methods. Neurogastroenterol Motil. 2000;12:361–8.
De Giorgio R, Barbara G. Is irritable bowel syndrome an inflammatory disorder? Curr Gastroenterol Rep. 2008;10:385–90.
Kindt S, Van Oudenhove L, Broekaert D, et al. Immune dysfunction in patients with functional gastrointestinal disorders. Neurogastroenterol Motil. 2009;21:389–98.
Beatty JK, Bhargava A, Buret AG. Post-infectious irritable bowel syndrome: mechanistic insights into chronic disturbances following enteric infection. World J Gastroenterol. 2014;20:3976–85.
Acknowledgments
This work was supported in part by Grants-in-Aid from the Ministry of Education, Culture, Sports, Science, and Technology of Japan [C-23590252 (TN), and C-22590753 (TO)].
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nozu, T., Kumei, S., Miyagishi, S. et al. Colorectal distention induces acute and delayed visceral hypersensitivity: role of peripheral corticotropin-releasing factor and interleukin-1 in rats. J Gastroenterol 50, 1153–1161 (2015). https://doi.org/10.1007/s00535-015-1070-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-015-1070-3