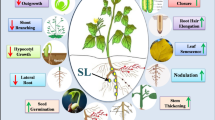
Abstract
Main conclusions
Exogenously applied GR24 affected somatic embryo formation and morphogenesis of strigolactone-deficient tomato mutant through cross-talk with auxins and cytokinins indicating involvement of SLs in the embryogenic process.
Strigolactones (SLs) mediate the regulation of plant responses to the environment through cross-talk with other plant hormones, especially auxins. Auxins play a crucial role in coordinating the morphogenesis and development of plant reproductive organs, including the signal-transduction cascade leading to the reprogramming of gene-expression patterns before embryo formation. SLs’ role in these processes is unknown, in contrast to their proven involvement in auxin transport and distribution. We used tomato cv. M82 and its SL-deficient mutant SL-ORT1 to study the influence of SLs on hormone profile in tomato roots and shoots, and their involvement in somatic embryogenesis (SE) and morphogenesis (adventitious root formation). The synthetic SL GR24 had different effects on SE of M82 and SL-ORT1, indicating that SLs influence the cytokinin-to-auxin ratio in tomato SE.





Similar content being viewed by others
Abbreviations
- 6-BA:
-
6-Benzylaminopurine
- BM:
-
Basal medium
- 2,4-D:
-
2,4-Dichlorophenoxyacetic acid
- IAA:
-
Indolelacetic acid
- MS:
-
Murashige and Skoog
- SL:
-
Strigolactone
- SE:
-
Somatic embryogenesis
References
Agusti J, Herold S, Schwarz M et al (2011) Strigolactone signaling is required for auxin-dependent stimulation of secondary growth in plants. Proc Natl Acad Sci USA 108:20242–20247
Akiyama K, Matsuzaki K, Hayashi H (2005) Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature 435:824–827
Alder A, Jamil M, Marzorati M, Bruno M, Vermathen M, Bigler P, Ghisla S, Bouwmeester H, Beyer P, Al-Babili S (2012) The path from β-carotene to carlactone, a strigolactone-like plant hormone. Science 335:1348–1351
Andreo-Jimenez B, Ruyter-Spira C, Bouwmeester HJ, Lopez-Raez JA (2015) Ecological relevance of strigolactones in nutrient uptake and other abiotic stresses, and in plant-microbe interactions below-ground. Plant Soil 394:1–19
Benková E, Michniewicz M, Sauer M, Teichmann T, Seifertova D, Jürgens G, Friml J (2003) Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell 115:591–602
Bernardi J, Lanubile A, Li Q-B, Kumar D, Kladnik A, Cook SD, Ross JJ, Marocco A, Chourey PS (2012) Impaired auxin biosynthesis in the defective endosperm18 mutant is due to mutational loss of expression in the ZmYuc1 gene encoding endosperm-specific YUCCA1 protein in maize. Plant Physiol 160:1318–1328
Beveridge CA (2006) Axillary bud outgrowth: sending a message. Curr Opin Plant Biol 9:35–40
Bhatia P, Nanjappa A, Tissa S, David M (2004) Tissue culture studies in tomato (Lycopersicon esculentum). Plant Cell Tiss Org 78:1–21
Braun N, de Saint Germain A, Pillot J-P et al (2012) The pea TCP transcription factor PsBRC1 acts downstream of strigolactones to control shoot branching. Plant Physiol 158:225–238
Chandel G, Katiyar SK (2000) Organogenesis and somatic embryogenesis in tomato (Lycopersicon esculantum Mill.). Adv Plant Sci 13:11–17
Chen LZ, Adachi T (1998) Protoplast fusion between Lycopersicon esculentum and L. peruvianum-complex: somatic embryogenesis, plant regeneration and morphology. Plant Cell Rep 17:508–514
Cheng Y, Dai X, Zhao Y (2007) Auxin synthesized by the YUCCA flavin monooxygenases is essential for embryogenesis and leaf formation in Arabidopsis. Plant Cell 19:2430–2439
Chiou T-J, Lin S-I (2011) Signaling network in sensing phosphate availability in plants. Annu Rev Plant Biol 62:185–206
Dor E, Alperin B, Wininger S, Ben-Dor B, Somvanshi VS, Koltai H, Kapulnik Y, Hershenhorn J (2010) Characterization of a novel tomato mutant resistant to Orobanche and Phelipanche spp. weedy parasites. Euphytica 171:371–380
Dor E, Yoneyama K, Wininger S, Kapulnik Y, Yoneyama K, Koltai H, Xie X, Hershenhorn J (2011) Strigolactone deficiency confers resistance in tomato line SL-ORT1 to the parasitic weeds Phelipanche and Orobanche spp. Phytopathology 101:213–222
Dun EA, De Saint Germain A, Rameau C, Beveridge CA (2012) Antagonistic action of strigolactone and cytokinin in bud outgrowth control. Plant Physiol 158:487–498
Eshed Y, Abu-Abied M, Saranga Y, Zamir D (1992) Lycopersicon esculentum lines containing small overlappping introgressions from L. pennellii. Theor Appl Genet 83:1027–1034
Fehér A (2005) Why somatic plant cells start to form embryos? In: Mujib A, Samaj J (eds.) Somatic embryogenesis. Plant Cell Monographs, vol 2. Springer-Verlag, Berlin, pp 85–101
Fehér A, Taras P, Pasternak T, Dudits D (2003) Transition of somatic plant cells to an embryogenic state. Plant Cell Tiss Org 74:201–228
Gaj MD (2004) Factors influencing somatic embryogenesis induction and plant regeneration with particular reference to Arabidopsis thaliana (L.) Heynh. Plant Growth Regul 43:27–47
Gill R, Malik KA, Sanago MHM, Saxena PK (1995) Somatic embryogenesis and plant regeneration from seedling cultures of tomato (Lycopersicon esculentum Mill.). J Plant Physiol 147:273–276
Gliwicka M, Nowak K, Balazadeh S, Mueller-Roeber B, Gaj MD (2013) Extensive modulation of the transcription factor transcriptome during somatic embryogenesis in Arabidopsis thaliana. PLoS One 8:e69261
Gomez-Roldan V, Fermas S, Brewer PB et al (2008) Strigolactone inhibition of shoot branching. Nature 455:189–194
Guan ZJ, Guo B, Huo YL, Guan ZP, Dai JK, Wei YH (2012) Short communication: organogenesis and somatic embryogenesis in callus derived from HBsAg-transgenic tomato mutant. Can J Plant Sci 92:747–756
Hayward A, Stirnberg P, Beveridge C, Leyser O (2009) Interactions between auxin and strigolactone in shoot branching control. Plant Physiol 151:400–412
Jin F, Hu L, Yuan D, Xu J, Gao W, He L, Yang X, Zhang X (2014) Comparative transcriptome analysis between somatic embryos (SEs) and zygotic embryos in cotton: evidence for stress response functions in SE development. Plant Biotech J 12:161–173
Johnson X, Brcich T, Dun EA, Goussot M, Haurogne K, Beveridge CA, Rameau C (2006) Branching genes are conserved across species. Genes controlling a novel signal in pea are coregulated by other long-distance signals. Plant Physiol 142:1014–1026
Joshi R, Kumar P (2013) Regulation of somatic embryogenesis in crops: a review. Agric Rev 34:1–21
Kapulnik Y, Delaux P-M, Resnick N et al (2011a) Strigolactones affect lateral root formation and root-hair elongation in Arabidopsis. Planta 233:209–216
Kapulnik Y, Resnick N, Mayzlish-Gati E, Kaplan Y, Wininger S, Hershenhorn J, Koltai H (2011b) Strigolactones interact with ethylene and auxin in regulating root-hair elongation in Arabidopsis. J Exp Bot 62:2915–2924
Khuong TTH, Crété P, Robaglia C, Caffarri S (2013) Optimization of tomato micro-tom regeneration and selection on glufosinate/Basta and dependency of gene silencing on transgene copy number. Plant Cell Rep 32:1441–1454
Kohlen W, Charnikhova T, Lammers M et al (2012) The tomato CAROTENOID CLEAVAGE DIOXYGENASE8 (SlCCD8) regulates rhizosphere signaling, plant architecture and affects reproductive development through strigolactone biosynthesis. New Phytol 196:535–547
Koltai H (2014) Receptors, repressors, PINs: a playground for strigolactone signaling. Trends Plant Sci 19:727–733
Koltai H (2015) Cellular events of strigolactone signalling and their crosstalk with auxin in roots. J Exp Bot 66:4855–4861
Koltai H, Kapulnik Y (2011) Strigolactones as mediators of plant growth responses to environmental conditions. Plant Signal Behav 6:37–41
Koltai H, Dor E, Hershenhorn J et al (2010a) Strigolactones’ effect on root growth and root-hair elongation may be mediated by auxin-efflux carriers. J Plant Growth Regul 29:129–136
Koltai H, LekKala SP, Bhattacharya C, Mayzlish-Gati E, Resnick N, Wininger S, Dor E, Kaori Yoneyama, Koichi Yoneyama, Hershenhorn J, Joel DM, Kapulnik Y (2010b) A tomato strigolactone -impaired mutant displays aberrant shoot morphology and plant interactions. J Exp Bot 61:1739–1749
Leljak-Levanić D, Mihaljević S, Bauer N (2015) Somatic and zygotic embryos share common developmental features at the onset of plant embryogenesis. Acta Physiol Plant 37:127
Li S, Xue L, Xu S, Feng H, An L (2009) Mediators, genes and signaling in adventitious rooting. Bot Rev 75:230–247
Liang J, Zhao L, Challis R, Leyser O (2010) Strigolactone regulation of shoot branching in chrysanthemum (Dendranthema grandiflorum). J Exp Bot 61:3069–3078
Ljung K, Bhalerao RP, Sandberg G (2001) Sites and homeostatic control of auxin biosynthesis in Arabidopsis during vegetative growth. Plant J 28:465–474
Mashiguchi K, Sasaki E, Shimada Y, Nagae M, Ueno K, Nakano T, Yoneyama K, Suzuki Y, Asami T (2009) Feedback-regulation of strigolactone biosynthetic genes and strigolactone-related genes in Arabidopsis. Biosci Biotechnol Biochem 73:2460–2465
Nakamura H, Xue YL, Miyakawa T, et al. (2013) Molecular mechanism of strigolactone perception by DWARF14. Nature Communications 4 (article number 2613)
Negi S, Sukumar P, Liu X, Cohen JD, Muday GK (2010) Genetic dissection of the role of ethylene in regulating auxin-dependent lateral and adventitious root formation in tomato. Plant J 61:3–15
Nishiwaki M, Fujino K, Koda Y, Masuda K, Kikuta Y (2000) Somatic embryogenesis induced by the simple application of abscisic acid to carrot (Daucus carota L.) seedlings in culture. Planta 211:756–759
Pandya-Kumar N, Shema R, Kumar M et al (2014) Strigolactone analog GR24 triggers changes in PIN2 polarity, vesicle trafficking and actin filament architecture. New Phytol 202:1184–1196
Proust H, Hoffmann B, Xie X, Yoneyama K, Schaefer DG, Yoneyama K, Nogué F, Rameau C (2011) Strigolactones regulate protonema branching and act as a quorum sensing-like signal in the moss Physcomitrella patens. Development 138:1531–1539
Rai GK, Rai NP, Kumar S, Yadav A, Rathaur S, Singh M (2012) Effects of explant age, germination medium, pre-culture parameters, inoculation medium, pH, washing medium, and selection regime on Agrobacterium-mediated transformation of tomato. In Vitro Cell Dev Biol Plant 48:565–578
Rasmussen A, Mason M, De Cuyper C et al (2012) Strigolactones suppress adventitious rooting in Arabidopsis and pea. Plant Physiol 158:1976–1987
Sang D, Chen D, Liu G, Liang Y, Huang L, Meng X, Chu J, Sun X, Dong G, Yuan Y (2014) Strigolactones regulate rice tiller angle by attenuating shoot gravitropism through inhibiting auxin biosynthesis. Proc Natl Acad Sci USA 111:11199–11204
Seto Y, Kameoka H, Yamaguchi S, Kyozuka J (2012) Recent advances in strigolactone research: chemical and biological aspects. Plant Cell Physiol 53:1843–1853
Sharp WR, Sondahl MR, Caldas LS, Maraffa SB (1980) The physiology of in vitro asexual embryogenesis. Hort Rev 2:268–310
Shinohara N, Taylor C, Leyser O (2013) Strigolactone can promote or inhibit shoot branching by triggering rapid depletion of the auxin efflux protein PIN1 from the plasma membrane. PLoS Biol 11:e1001474
Snowden KC, Simkin AJ, Janssen BJ, Templeton KR, Loucas HM, Simons JL, Karunairetnam S, Gleave AP, Clark DG, Klee HJ (2005) The Decreased apical dominance1/Petunia hybrida CAROTENOID CLEAVAGE DIOXYGENASE8 gene affects branch production and plays a role in leaf senescence, root growth, and flower development. Plant Cell 17:746–759
Stepanova AN, Robertson-Hoyt J, Yun JJ, Benavente LM, Xie D-Y, Dolezal K, Schlereth A, Jürgens G, Alonso JM (2008) TAA1- mediated auxin biosynthesis is essential for hormone crosstalk and plant development. Cell 133:177–191
Toh S, Kamiya Y, Kawakami N, Nambara E, McCourt P, Tsuchiya Y (2012) Thermoinhibition uncovers a role for strigolactones in Arabidopsis seed germination. Plant Cell Physiol 53:107–117
Tsuchiya Y, McCourt P (2009) Strigolactones: a new hormone with a past. Curr Opin Plant Biol 12:556–561
Tsuchiya Y, Vidaurre D, Toh S, Hanada A, Nambara E, Kamiya Y, Yamaguchi S, McCourt P (2010) A small-molecule screen identifies new functions for the plant hormone strigolactone. Nat Chem Biol 6:741–749
Umehara M, Hanada A, Yoshida S et al (2008) Inhibition of shoot branching by new terpenoid plant hormones. Nature 455:195–200
Waldie T, McCulloch H, Leyser O (2014) Strigolactones and the control of plant development: lessons from shoot branching. Plant J 79:607–622
Wang Y, Sun S, Zhu W, Jia K, Yang H, Wang X (2013) Strigolactone/MAX2- induced degradation of brassinosteroid transcriptional effector BES1 regulates shoot branching. Dev Cell 27:681–688
Xie X, Yoneyama K, Yoneyama K (2010) The strigolactone story. Annu Rev Phytopathol 48:93–117
Yamada Y, Furusawa S, Nagasaka S, Shimomura K, Yamaguchi S, Umehara M (2014) Strigolactone signaling regulates rice leaf senescence in response to a phosphate deficiency. Planta 240:399–408
Yoneyama K, Xie X, Kim H, Kisugi T, Nomura T, Sekimoto H, Yokota T, Yoneyama K (2012) How do nitrogen and phosphorus deficiencies affect strigolactone production and exudation? Planta 235:1197–1207
Yoneyama K, Kisugi T, Xie X, Yoneyama K (2013) Chemistry of strigolactones: why and how do plants produce so many strigolactones? In: de Bruijn FJ (ed) Molecular microbial ecology of the rhizosphere. Wiley, Hoboken, pp 373–379
Young NF, Ferguson BJ, Antoniadi I, Bennett MH, Beveridge CA, Turnbull CG (2014) Conditional auxin response and differential cytokinin profiles in shoot branching mutants. Plant Physiol 165:1723–1736
Zwanenburg B, Pospíšil T (2013) Structure and activity of strigolactones: new plant hormones with a rich future. Mol Plant 6:38–62
Acknowledgements
We thank the Ministry of Finance of the State of Israel for financial support of Dr. Yuanli Wu’s post doctoral fellowship at Newe Ya’ar Research Center, Israel, and Prof. Koichi Yoneyama for kindly providing us with GR24.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wu, Y., Dor, E. & Hershenhorn, J. Strigolactones affect tomato hormone profile and somatic embryogenesis. Planta 245, 583–594 (2017). https://doi.org/10.1007/s00425-016-2625-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-016-2625-0