Abstract
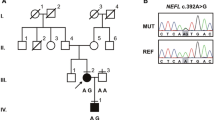
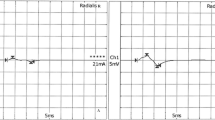
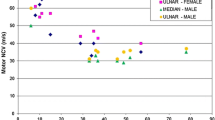
The purpose of the study was to describe a pedigree with NEFL E396K mutation associated with a novel dominant intermediate Charcot–Marie–Tooth disease (DI-CMT) phenotype. The pedigree comprised four patients over two generations, aged between 35 and 59 years, who have been serially evaluated since 1993. Their clinical picture was characterized by pes cavus, sensorimotor neuropathy and spastic gait. Both older patients showed ascending leg weakness to involve pelvic musculature. CMT neuropathy score ranged from 14 to 26 (moderate to severe disease). Electrophysiology showed uniform nerve conduction slowing in the intermediate range, both in distal and proximal nerve segments. Multimodal evoked potential and blink reflex studies revealed abnormalities indicative of central sensorimotor pathway dysfunction. On imaging studies of lower-limb musculature, there was massive atrophy of intrinsic foot muscles and to a lesser degree of calves and thighs predominating in muscles innervated by tibial and sciatic nerves. In both patients exhibiting waddling gait, there was atrophy of pelvic muscles mainly involving gluteus medius, gluteus minimus and piriformis. We conclude that NEFL E396K mutation may manifest with a novel DI-CMT phenotype, characterized by simultaneous involvement of the peripheral and central nervous system.







Similar content being viewed by others
References
Harding AE, Thomas PK (1980) The clinical features of hereditary motor and sensory neuropathy types I and II. Brain 103:259–280
Davis CJ, Bradley WG, Madrid R (1978) The peroneal muscular atrophy syndrome: clinical, genetic, electrophysiological and nerve biopsy studies. I. Clinical, genetic and electrophysiological findings and classification. J Genet Hum 26:311–349
Madrid R, Bradley WG, Davis CJ (1977) The peroneal muscular atrophy syndrome. Clinical, genetic, electrophysiological and nerve biopsy studies. Part 2. Observations on pathological changes in sural nerve biopsies. J Neurol Sci 32:91–122
Bouché P, Gherardi R, Cathala HP, Lhermitte F, Castaigne P (1983) Peroneal muscular atrophy. Part 1. Clinical and electrophysiological study. J Neurol Sci 61:389–399
Nicholson G, Myers S (2006) Intermediate forms of Charcot–Marie–Tooth neuropathy: a review. Neuromolecular Med 8:123–130
Rossor AM, Polke JM, Houlden H, Reilly MM (2013) Clinical implications of genetic advances in Charcot–Marie–Tooth disease. Nat Rev Neurol 9:562–571
Nicholson G, Nash J (1993) Intermediate nerve conduction velocities define X-linked Charcot–Marie–Tooth neuropathy families. Neurology 43:2558–2564
Liu L, Zhang R (2014) Intermediate Charcot–Marie–Tooth disease. Neurosci Bull 30:999–1009
Mersiyanova IV, Perepelov AV, Polyakov AV, Sitnikov VF, Dadali EL, Oparin RB, Petrin AN, Evgrafov OV (2009) A new variant of Charcot–Marie–Tooth disease type 2 is probably the result of a mutation in the neurofilament-light gene. Am J Hum Genet 67:37–46
De Jonghe P, Mersivanova I, Nelis E, Del Favero J, Martin JJ, Van Broeckhoven C, Evgrafov O, Timmerman V (2001) Further evidence that neurofilament light chain gene mutations can cause Charcot–Marie–Tooth disease type 2E. Ann Neurol 49:245–249
Georgiou DM, Zidar J, Korosec M, Middleton LT, Kyriakides T, Christodoulou K (2002) A novel NF-L mutation Pro22Ser is associated with CMT2 in a large Slovenian family. Neurogenetics 4:93–96
Jordanova A, De Jonghe P, Boerkoel CF, Takashima H, De Vriendt E, Ceuterick C, Martin JJ, Butler IJ, Mancias P, Papasozomenos SCh, Terespolsky D, Potocki L, Brown CW, Shy M, Rita DA, Tournev I, Kremensky I, Lupski JR, Timmerman V (2003) Mutations in the neurofilament light chain gene (NEFL) cause early onset severe Charcot–Marie–Tooth disease. Brain 126:590–597
Züchner S, Vorgerd M, Sindern E, Schröder JM (2004) The novel neurofilament light (NEFL) mutation Glu397Lys is associated with a clinically and morphologically heterogeneous type of Charcot–Marie–Tooth neuropathy. Neuromuscul Disord 14:147–157
Fabrizi GM, Cavallaro T, Angiari C, Cabrini I, Taioli F, Malerba G, Bertolasi L, Rizzuto N (2007) Charcot–Marie–Tooth disease type 2E, a disorder of the cytoskeleton. Brain 130:394–403
Yum SW, Zhang J, Mo K, Li J, Scherer SS (2009) A novel recessive Nefl mutation causes a severe, early-onset axonal neuropathy. Ann Neurol 66:759–770
Abe A, Numakura C, Saito K, Koide H, Oka N, Honma A, Kishikawa Y, Hayasaka K (2009) Neurofilament light chain polypeptide gene mutations in Charcot–Marie–Tooth disease: nonsense mutation probably causes a recessive phenotype. J Hum Genet 54:94–97
Miltenberger-Miltenyi G, Janecke AR, Wanschitz JV, Timmerman V, Windpassinger C, Auer-Grumbach M, Löscher WN (2007) Clinical and electrophysiological features in Charcot–Marie–Tooth disease with mutations in the NEFL gene. Arch Neurol 64:966–970
Lin KP, Soong BW, Yang CC, Huang LW, Chang MH, Lee IH, Antonellis A, Lee YC (2011) The mutational spectrum in a cohort of Charcot–Marie–Tooth disease type 2 among the Han Chinese in Taiwan. PLoS One 6:e29393
Saporta AS, Sottile SL, Miller LJ, Feely SM, Siskind CE, Shy ME (2011) Charcot–Marie–Tooth disease subtypes and genetic testing strategies. Ann Neurol 69:22–33
Murphy SM, Laura M, Fawcett K, Pandraud A, Liu YT, Davidson GL, Rossor AM, Polke JM, Castleman V, Manji H, Lunn MP, Bull K, Ramdharry G, Davis M, Blake JC, Houlden H, Reilly MM (2012) Charcot–Marie–Tooth disease: frequency of genetic subtypes and guidelines for genetic testing. J Neurol Neurosurg Psychiatry 83:706–710
Sivera R, Sevilla T, Vílchez JJ, Martínez-Rubio D, Chumillas MJ, Vázquez JF, Muelas N, Bataller L, Millán JM, Palau F, Espinós C (2013) Charcot–Marie–Tooth disease: genetic and clinical spectrum in a Spanish clinical series. Neurology 81:1617–1625
García A, Combarros O, Calleja J, Berciano J (1998) Charcot–Marie–Tooth disease type 1A with 17p duplication in infancy and early childhood: a longitudinal clinical and electrophysiologic study. Neurology 5:1061–1067
Berciano J, Gallardo E, García A, Infante J, Mateo I, Combarros O (2006) Charcot–Marie–Tooth disease type 1A duplication with severe paresis of the proximal lower limb muscles: a long-term follow-up study. J Neurol Neurosurg Psychiatry 77:1169–1176
Shy ME, Blake J, Krajewski K, Fuerst DR, Laura M, Hahn AF, Li J, Lewis RA, Reilly M (2005) Reliability and validity of the CMT neuropathy score as a measure of disability. Neurology 64:1209–1214
García A, Pelayo-Negro AL, Alvarez-Paradelo S, Antolín FM, Berciano J (2015) Electromyographic tendon reflex recording: an accurate and comfortable method for diagnosis of Charcot–Marie–Tooth disease type 1A. Muscle Nerve. doi:10.1002/mus.24499 (Epub ahead of print)
Chiappa KH (1997) Evoked potentials in clinical medicine, 3rd edn. Lippincott-Raven Press Publishers, Philadelphia-New York
Gallardo E, García A, Combarros O, Berciano J (2006) Charcot–Marie–Tooth disease type 1A duplication: spectrum of clinical and magnetic resonance imaging features in leg and foot muscles. Brain 129:426–437
Reumers J, De Rijk P, Zhao H, Liekens A, Smeets D, Cleary J, Van Loo P, Van Den Bossche M, Catthoor K, Sabbe B, Despierre E, Vergote I, Hilbush B, Lambrechts D, Del-Favero J (2011) Optimized filtering reduces the error rate in detecting genomic variants by short-read sequencing. Nat Biotechnol 18(30):61
McKenna A, Hanna M, Banks E, Sivachenko A, Cibulskis K, Kernytsky A, Garimella K, Altshuler D, Gabriel S, Daly M, DePristo MA (2010) The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res 20:1297–1303
Elbracht M, Senderek J, Schara U, Nolte K, Klopstock T, Roos A, Reimann J, Zerres K, Weis J, Rudnik-Schöneborn S (2014) Clinical and morphological variability of the E396K mutation in the neurofilament light chain gene in patients with Charcot–Marie–Tooth disease type 2E. Clin Neuropathol 33:335–343
Harding AE, Thomas PK (1984) Peroneal muscular atrophy with pyramidal features. J Neurol Neurosurg Psychiatry 47:168–172
Hashiguchi A, Higuchi Y, Nomura M, Nakamura T, Arata H, Yuan J, Yoshimura A, Okamoto Y, Matsuura E, Takashima H (2014) Neurofilament light mutation causes hereditary motor and sensory neuropathy with pyramidal signs. J Peripher Nerv Syst 19:311–316
Buchthal F, Behse F (1977) Peroneal muscular atrophy (PMA) and related disorders. I. Clinical manifestations as related to biopsy findings, nerve conduction and electromyography. Brain 100:41–66
Pelayo-Negro AL, Carr AS, Laura M, Skorupinska M, Reilly MM (2015) An observational study of asymmetry in CMT1A. J Neurol Neurosurg Psychiatry. doi:10.1136/jnnp-2014-309096 (Epub ahead of print)
Leung CL, Nagan N, Graham TH, Liem RK (2006) A novel duplication/insertion mutation of NEFL in a patient with Charcot–Marie–Tooth disease. Am J Med Genet A 140:1021–1025
Agrawal PB, Joshi M, Marinakis NS, Schmitz-Abe K, Ciarlini PD, Sargent JC, Markianos K, De Girolami U, Chad DA, Beggs AH (2014) Expanding the phenotype associated with the NEFL mutation: neuromuscular disease in a family with overlapping myopathic and neurogenic findings. JAMA Neurol 71:1413–1420
Choi BO, Lee MS, Shin SH, Hwang JH, Choi KG, Kim WK, Sunwoo IN, Kim NK, Chung KW (2004) Mutational analysis of PMP22, MPZ, GJB1, EGR2 and NEFL in Korean Charcot–Marie–Tooth neuropathy patients. Hum Mutat 24:185–186
Cooper DN, Youssoufian H (1988) The CpG dinucleotide and human genetic disease. Hum Genet 78:151–155
Banchs I, Casasnovas C, Montero J, Volpini V, Martínez-Matos JA (2010) Charcot–Marie–Tooth disease with intermediate conduction velocities caused by a novel mutation in the MPZ gene. Muscle Nerve 42:184–188
Shin JS, Chung KW, Cho SY, Yun J, Hwang SJ, Kang SH, Cho EM, Kim SM, Choi BO (2008) NEFL Pro22Arg mutation in Charcot–Marie–Tooth disease type 1. J Hum Genet 53:936–940
Lee SS, Lee HJ, Park JM, Hong YB, Park KD, Yoo JH, Koo H, Jung SC, Park HS, Lee JH, Lee MG, Hyun YS, Nakhro K, Chung KW, Choi BO (2013) Proximal dominant hereditary motor and sensory neuropathy with proximal dominance association with mutation in the TRK-fused gene. JAMA Neurol 70:607–615
Shen H, Barry DM, Dale JM, Garcia VB, Calcutt NA, Garcia ML (2011) Muscle pathology without severe nerve pathology in a new mouse model of Charcot–Marie–Tooth disease type 2E. Hum Mol Genet 20:2535–2548
Chung KW, Suh BC, Shy ME, Cho SY, Yoo JH, Park SW, Moon H, Park KD, Choi KG, Kim S, Kim SB, Shim DS, Kim SM, Sunwoo IN, Choi BO (2008) Different clinical and magnetic resonance imaging features between Charcot–Marie–Tooth disease type 1A and 2A. Neuromuscul Disord 18:610–618
Gaeta M, Mileto A, Mazzeo A, Minutoli F, Di Leo R, Settineri N, Donato R, Ascenti G, Blandino A (2012) MRI findings, patterns of disease distribution, and muscle fat fraction calculation in five patients with Charcot–Marie–Tooth type 2F disease. Skeletal Radiol 41:515–524
Kamath S, Venkatanarasimha N, Walsh MA, Hughes PM (2008) MRI appearance of muscle denervation. Skeletal Radiol 37:397–404
Davis DV, Coupland RE (1967) Gray’s anatomy, Descriptive and applied. Longmans, Green & Co. LTD, London
Kim HK, Merrow AC, Shiraj S, Wong BL, Horn PS, Laor T (2013) Analysis of fatty infiltration and inflammation of the pelvic and thigh muscles in boys with Duchenne muscular dystrophy (DMD): grading of disease involvement on MR imaging and correlation with clinical assessments. Pediatr Radiol 43:1327–1335
Faridian-Aragh N, Wagner KR, Leung DG, Carrino JA (2014) Magnetic resonance imaging phenotyping of Becker muscular dystrophy. Muscle Nerve 50:962–967
de Visser M, Verbeeten B Jr (1985) Computed tomography of the skeletal musculature in Becker-type muscular dystrophy and benign infantile spinal muscular atrophy. Muscle Nerve 8:435–444
De Pablos C, Berciano J, Calleja J (1991) Brain-stem auditory evoked potentials and blink reflex in Friedreich’s ataxia. J Neurol 238:212–216
Perrot R, Berges R, Bocquet A, Eyer J (2008) Review of the multiple aspects of neurofilament functions, and their possible contribution to neurodegeneration. Mol Neurobiol 38:27–65
Liem RK, Messing A (2009) Dysfunctions of neuronal and glial intermediate filaments in disease. J Clin Invest 119:1814–1824
Perez-Olle R, Leung CL, Liem RK (2002) Effects of Charcot–Marie–Tooth-linked mutations of the neurofilament light subunit on intermediate filament formation. J Cell Sci 115:4937–4946
Pérez-Ollé R, López-Toledano MA, Goryunov D, Cabrera-Poch N, Stefanis L, Brown K, Liem RK (2005) Mutations in the neurofilament light gene linked to Charcot–Marie–Tooth disease cause defects in transport. J Neurochem 93:861–874
Crimella C, Baschirotto C, Arnoldi A, Tonelli A, Tenderini E, Airoldi G, Martinuzzi A, Trabacca A, Losito L, Scarlato M, Benedetti S, Scarpini E, Spinicci G, Bresolin N, Bassi MT (2012) Mutations in the motor and stalk domains of KIF5A in spastic paraplegia type 10 and in axonal Charcot–Marie–Tooth type 2. Clin Genet 82:157–164
Adebola AA, Di Castri T, He C, Salvatierra LA, Zhao J, Brown K, Lin C, Worman HJ, Liem RK (2015) Neurofilament light polypeptide gene N98S mutation in mice leads to neurofilament network abnormalities and a Charcot–Marie–Tooth Type 2E phenotype. Hum Mol Genet 24:2163–2174
Acknowledgments
We thank our patients for their collaboration, without which this study would not have been possible. The study was supported by Research Institute of University Hospital “Marqués de Valdecilla” (IDIVAL) Grants BFR 05/10 and WLA 03/12. The Antwerp team was funded in part by the University of Antwerp (TOP BOF 29069); the Fund for Scientific Research-Flanders (FWO) and the Belgian Association against Neuromuscular Disorders (ABMM). KP is supported by a PhD fellowship from the Fund for Scientific Research-Flanders (FWO).
Conflicts of interest
We declare no conflicts of interest.
Ethical standard
Ethical approval was obtained and all patients and at-risk subjects, and control subjects gave informed consent for this study. This investigation was approved by the “Comité Ético de Investigación Clínica de Cantabria”.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
415_2015_7709_MOESM1_ESM.mpg
Supplementary material, video 1. This video illustrates serial clinical signs in patient III-1 (the proband). Segment 1: In 2004 (at age 29), there was lower-leg wasting slightly more marked on the left side. Note normal appearance of both thighs. Segment 2: Ten years later note progression of lower-leg amyotrophy. Segment 3: Gait in 2004 was stepping, waddling and spastic; tiptoe walking was difficult and heel walking almost impossible. Segment 4: Ten years later, there was marked progression of gait abnormalities. Segment 5: Mild hand amyotrophy with wasting of the first dorsal interossei and flattening of thenar eminences. Segment 6: Ten years later note evident progression of hand amyotrophy, now with incipient and bilateral clawing of the third to fifth fingers. Segment 7: In 2014, there was neither amyotrophy nor weakness of proximal upper-limb musculature. (MPG 13204 kb)
415_2015_7709_MOESM2_ESM.mpg
Supplementary material, video 2. This video illustrates serial clinical signs in patient II-4. Segment 1: In 2004 (at age 49), there was lower-leg wasting minimally more marked on the left side. Note normal appearance of both thighs. Segment 2: Ten years later stance is possible only with support; lower-limb amyotrophy is now masked by weight gain. Segment 3: Gait in 2004 was stepping, waddling and spastic; tiptoe walking was difficult and heel walking impossible. Segment 4: Ten years later, there was marked progression of gait abnormalities, the patient requiring support for walking. Segment 5: In 2004 note evident wasting of the first dorsal interossei and flattening of thenar eminences. Segment 6: Ten years later note evident progression of hand amyotrophy. Segment 7: Plantar reflexes were always extensor; this segment illustrates the left plantar reflex. (MPG 13524 kb)
Rights and permissions
About this article
Cite this article
Berciano, J., García, A., Peeters, K. et al. NEFL E396K mutation is associated with a novel dominant intermediate Charcot–Marie–Tooth disease phenotype. J Neurol 262, 1289–1300 (2015). https://doi.org/10.1007/s00415-015-7709-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-015-7709-4
Keywords
- Blink reflex
- Brainstem auditory evoked potentials
- Charcot–Marie–Tooth disease types 1F and 2E
- CMTNS
- Dominant intermediate Charcot–Marie–Tooth disease
- Electrophysiology
- Motor evoked potentials
- MRI
- Multi-slice CT
- Muscle fatty atrophy
- Muscle oedema
- NEFL E396K mutation
- Nerve conduction
- Neurofilament
- Pelvic girdle weakness
- Somatosensory evoked potentials
- SPG10
- T-reflex recording
- Visual evoked potentials