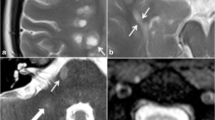
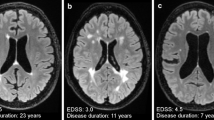
Abstract
In this review, we summarize advances in knowledge derived from the application of magnetic resonance imaging (MRI)-based techniques to patients with multiple sclerosis (MS) published in the Journal of Neurology over the past year. We highlight the pivotal role played by conventional MRI techniques for a correct and early diagnosis of this condition and the exclusion of alternative disorders. Advanced MR methods have contributed to demonstrating how damage to selected brain structures is related to disease clinical manifestations, thus contributing to overcome the well-known “clinical-radiological” paradox of MS, and ameliorating the understanding of the mechanisms underlying the accumulation of irreversible disability. Finally, we discuss the use of MRI to assess treatment efficacy and optimize therapeutic approaches in this condition.
Similar content being viewed by others
References
The Optic Neuritis Study Group (2008) Multiple sclerosis risk after optic neuritis: final optic neuritis treatment trial follow-up. Arch Neurol 65:727–732
Amato MP, Portaccio E, Goretti B, Zipoli V, Battaglini M, Bartolozzi ML, Stromillo ML, Guidi L, Siracusa G, Sorbi S, Federico A, De Stefano N (2007) Association of neocortical volume changes with cognitive deterioration in relapsing-remitting multiple sclerosis. Arch Neurol 64:1157–1161
Barkhof F, Filippi M, Miller DH, Tofts P, Kappos L, Thompson AJ (1997) Strategies for optimizing MRI techniques aimed at monitoring disease activity in multiple sclerosis treatment trials. J Neurol 244:76–84
Batista S, Zivadinov R, Hoogs M, Bergsland N, Heininen-Brown M, Dwyer MG, Weinstock-Guttman B, Benedict RH (2012) Basal ganglia, thalamus and neocortical atrophy predicting slowed cognitive processing in multiple sclerosis. J Neurol 259:139–146
Bot JC, Barkhof F (2009) Spinal-cord MRI in multiple sclerosis: conventional and nonconventional MR techniques. Neuroimaging Clin N Am 19:81–99
Calabrese M, Agosta F, Rinaldi F, Mattisi I, Grossi P, Favaretto A, Atzori M, Bernardi V, Barachino L, Rinaldi L, Perini P, Gallo P, Filippi M (2009) Cortical lesions and atrophy associated with cognitive impairment in relapsing-remitting multiple sclerosis. Arch Neurol 66:1144–1150
Charil A, Yousry TA, Rovaris M, Barkhof F, De Stefano N, Fazekas F, Miller DH, Montalban X, Simon JH, Polman C, Filippi M (2006) MRI and the diagnosis of multiple sclerosis: expanding the concept of “no better explanation”. Lancet Neurol 5:841–852
Chiaravalloti ND, DeLuca J (2008) Cognitive impairment in multiple sclerosis. Lancet Neurol 7:1139–1151
Dalton CM, Miszkiel KA, Barker GJ, MacManus DG, Pepple TI, Panzara M, Yang M, Hulme A, O’Connor P, Miller DH (2004) Effect of natalizumab on conversion of gadolinium enhancing lesions to T1 hypointense lesions in relapsing multiple sclerosis. J Neurol 251:407–413
Eckstein C, Saidha S, Levy M (2012) A differential diagnosis of central nervous system demyelination: beyond multiple sclerosis. J Neurol 259:801–816
Filippi M, Agosta F, Spinelli EG, Rocca MA (2012) Imaging resting state brain function in multiple sclerosis. J Neurol. doi:10.1007/s00415-012-6695-z
Filippi M, Horsfield MA, Ader HJ, Barkhof F, Bruzzi P, Evans A, Frank JA, Grossman RI, McFarland HF, Molyneux P, Paty DW, Simon J, Tofts PS, Wolinsky JS, Miller DH (1998) Guidelines for using quantitative measures of brain magnetic resonance imaging abnormalities in monitoring the treatment of multiple sclerosis. Ann Neurol 43:499–506
Filippi M, Rocca MA, Camesasca F, Cook S, O’Connor P, Arnason BG, Kappos L, Goodin D, Jeffery D, Hartung HP, Comi G, Wolinsky JS, Bogumil T, Pohl C, Beckmann K, Sandbrink R, Croze E, Brown C, Desimone TM, Arnold DL, Cutter G, Knappertz V (2011) Interferon beta-1b and glatiramer acetate effects on permanent black hole evolution. Neurology 76:1222–1228
Filippi M, Rocca MA, Colombo B, Falini A, Codella M, Scotti G, Comi G (2002) Functional magnetic resonance imaging correlates of fatigue in multiple sclerosis. Neuroimage 15:559–567
Filippi M, Rocca MA, De Stefano N, Enzinger C, Fisher E, Horsfield MA, Inglese M, Pelletier D, Comi G (2011) Magnetic resonance techniques in multiple sclerosis: the present and the future. Arch Neurol 68:1514–1520
Filippi M, Rovaris M, Rocca MA, Sormani MP, Wolinsky JS, Comi G (2001) Glatiramer acetate reduces the proportion of new MS lesions evolving into “black holes”. Neurology 57:731–733
Goodin DS (2006) Magnetic resonance imaging as a surrogate outcome measure of disability in multiple sclerosis: have we been overly harsh in our assessment? Ann Neurol 59:597–605
Hackmack K, Weygandt M, Wuerfel J, Pfueller CF, Bellmann-Strobl J, Paul F, Haynes JD (2012) Can we overcome the ‘clinico-radiological paradox’ in multiple sclerosis? J Neurol 259:2151–2160
Harrison DM, Shiee N, Bazin PL, Newsome SD, Ratchford JN, Pham D, Calabresi PA, Reich DS (2012) Tract-specific quantitative MRI better correlates with disability than conventional MRI in multiple sclerosis. J Neurol. doi:10.1007/s00415-012-6638-8
Havla J, Gerdes LA, Meinl I, Krumbholz M, Faber H, Weber F, Pellkofer HL, Hohlfeld R, Kumpfel T (2011) De-escalation from natalizumab in multiple sclerosis: recurrence of disease activity despite switching to glatiramer acetate. J Neurol 258:1665–1669
Havrdova E, Galetta S, Hutchinson M, Stefoski D, Bates D, Polman CH, O’Connor PW, Giovannoni G, Phillips JT, Lublin FD, Pace A, Kim R, Hyde R (2009) Effect of natalizumab on clinical and radiological disease activity in multiple sclerosis: a retrospective analysis of the Natalizumab Safety and Efficacy in Relapsing-Remitting Multiple Sclerosis (AFFIRM) study. Lancet Neurol 8:254–260
Hayton T, Furby J, Smith KJ, Altmann DR, Brenner R, Chataway J, Hunter K, Tozer DJ, Miller DH, Kapoor R (2012) Clinical and imaging correlates of the multiple sclerosis impact scale in secondary progressive multiple sclerosis. J Neurol 259:237–245
Hayton T, Furby J, Smith KJ, Altmann DR, Brenner R, Chataway J, Hunter K, Tozer DJ, Miller DH, Kapoor R (2012) Longitudinal changes in magnetisation transfer ratio in secondary progressive multiple sclerosis: data from a randomised placebo controlled trial of lamotrigine. J Neurol 259:505–514
Hellwig K, Gold R (2011) Progressive multifocal leukoencephalopathy and natalizumab. J Neurol 258:1920–1928
Hickman SJ, Dalton CM, Miller DH, Plant GT (2002) Management of acute optic neuritis. Lancet 360:1953–1962
Horwitz H, Degn M, Modvig S, Larsson HB, Wanscher B, Frederiksen JL (2012) CSF abnormalities can be predicted by VEP and MRI pathology in the examination of optic neuritis. J Neurol 259:2616–2620
Kapoor R, Furby J, Hayton T, Smith KJ, Altmann DR, Brenner R, Chataway J, Hughes RA, Miller DH (2010) Lamotrigine for neuroprotection in secondary progressive multiple sclerosis: a randomised, double-blind, placebo-controlled, parallel-group trial. Lancet Neurol 9:681–688
Kleinfeld K, Mobley B, Hedera P, Wegner A, Sriram S, Pawate S (2012) Adult-onset leukoencephalopathy with neuroaxonal spheroids and pigmented glia: report of five cases and a new mutation. J Neurol. doi:10.1007/s00415-012-6680-6
Kurtzke JF (1983) Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 33:1444–1452
Linda H, von Heijne A, Major EO, Ryschkewitsch C, Berg J, Olsson T, Martin C (2009) Progressive multifocal leukoencephalopathy after natalizumab monotherapy. N Engl J Med 361:1081–1087
Melin A, Outteryck O, Collongues N, Zephir H, Fleury MC, Blanc F, Lacour A, Ongagna JC, Berteloot AS, Vermersch P, de Seze J (2012) Effect of natalizumab on clinical and radiological disease activity in a French cohort of patients with relapsing-remitting multiple sclerosis. J Neurol 259:1215–1221
Miller DH, Weinshenker BG, Filippi M, Banwell BL, Cohen JA, Freedman MS, Galetta SL, Hutchinson M, Johnson RT, Kappos L, Kira J, Lublin FD, McFarland HF, Montalban X, Panitch H, Richert JR, Reingold SC, Polman CH (2008) Differential diagnosis of suspected multiple sclerosis: a consensus approach. Mult Scler 14:1157–1174
Nociti V, Batocchi AP, Luigetti M, Conte A, Lorusso VS, Roiati S, Tartaglione T, Del Grande A, Sabatelli M (2011) Progressive ascending myelopathy: atypical forms of multiple sclerosis or what else? J Neurol 258:1965–1970
Ormerod IE, Miller DH, McDonald WI, du Boulay EP, Rudge P, Kendall BE, Moseley IF, Johnson G, Tofts PS, Halliday AM et al (1987) The role of NMR imaging in the assessment of multiple sclerosis and isolated neurological lesions. A quant study. Brain 110(Pt 6):1579–1616
Paling D, Golay X, Wheeler-Kingshott C, Kapoor R, Miller D (2011) Energy failure in multiple sclerosis and its investigation using MR techniques. J Neurol 258:2113–2127
Papadopoulou A, von Felten S, Traud S, Rahman A, Quan J, King R, Garren H, Steinman L, Cutter G, Kappos L, Radue EW (2012) Evolution of MS lesions to black holes under DNA vaccine treatment. J Neurol 259:1375–1382
Pardini M, Bonzano L, Mancardi GL, Roccatagliata L (2010) Frontal networks play a role in fatigue perception in multiple sclerosis. Behav Neurosci 124:329–336
Patrucco L, Rojas JI, Cristiano E (2012) Assessing the value of spinal cord lesions in predicting development of multiple sclerosis in patients with clinically isolated syndromes. J Neurol 259:1317–1320
Polman CH, O’Connor PW, Havrdova E, Hutchinson M, Kappos L, Miller DH, Phillips JT, Lublin FD, Giovannoni G, Wajgt A, Toal M, Lynn F, Panzara MA, Sandrock AW (2006) A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med 354:899–910
Polman CH, Reingold SC, Banwell B, Clanet M, Cohen JA, Filippi M, Fujihara K, Havrdova E, Hutchinson M, Kappos L, Lublin FD, Montalban X, O’Connor P, Sandberg-Wollheim M, Thompson AJ, Waubant E, Weinshenker B, Wolinsky JS (2011) Diagnostic criteria for multiple sclerosis: 2010 Revisions to the McDonald criteria. Ann Neurol 69:292–302
Rao SM, Leo GJ, Bernardin L, Unverzagt F (1991) Cognitive dysfunction in multiple sclerosis. I. Frequency, patterns, and prediction. Neurology 41:685–691
Reilmann R, Holtbernd F, Bachmann R, Mohammadi S, Ringelstein EB, Deppe M (2012) Grasping multiple sclerosis: do quantitative motor assessments provide a link between structure and function? J Neurol. doi:10.1007/s00415-012-6639-7
Riccitelli G, Rocca MA, Forn C, Colombo B, Comi G, Filippi M (2011) Voxelwise assessment of the regional distribution of damage in the brains of patients with multiple sclerosis and fatigue. AJNR Am J Neuroradiol 32:874–879
Rieckmann P, Boyko A, Centonze D, Coles A, Elovaara I, Havrdova E, Hommes O, Lelorier J, Morrow SA, Oreja-Guevara C, Rijke N, Schippling S (2012) Future MS care: a consensus statement of the MS in the 21st Century Steering Group. J Neurol. doi:10.1007/s00415-012-6656-6
Rocca MA, Absinta M, Filippi M (2012) The role of advanced magnetic resonance imaging techniques in primary progressive MS. J Neurol 259:611–621
Rocca MA, Agosta F, Colombo B, Mezzapesa DM, Falini A, Comi G, Filippi M (2007) fMRI changes in relapsing-remitting multiple sclerosis patients complaining of fatigue after IFNbeta-1a injection. Hum Brain Mapp 28:373–382
Sepulcre J, Masdeu JC, Goni J, Arrondo G, Velez de Mendizabal N, Bejarano B, Villoslada P (2009) Fatigue in multiple sclerosis is associated with the disruption of frontal and parietal pathways. Mult Scler 15:337–344
Sicotte NL, Kern KC, Giesser BS, Arshanapalli A, Schultz A, Montag M, Wang H, Bookheimer SY (2008) Regional hippocampal atrophy in multiple sclerosis. Brain 131:1134–1141
Sormani MP, Bonzano L, Roccatagliata L, Cutter GR, Mancardi GL, Bruzzi P (2009) Magnetic resonance imaging as a potential surrogate for relapses in multiple sclerosis: a meta-analytic approach. Ann Neurol 65:268–275
Sormani MP, Bonzano L, Roccatagliata L, Mancardi GL, Uccelli A, Bruzzi P (2010) Surrogate endpoints for EDSS worsening in multiple sclerosis. A meta-anal approach. Neurol 75:302–309
Stuve O, Marra CM, Jerome KR, Cook L, Cravens PD, Cepok S, Frohman EM, Phillips JT, Arendt G, Hemmer B, Monson NL, Racke MK (2006) Immune surveillance in multiple sclerosis patients treated with natalizumab. Ann Neurol 59:743–747
Vellinga MM, Castelijns JA, Barkhof F, Uitdehaag BM, Polman CH (2008) Postwithdrawal rebound increase in T2 lesional activity in natalizumab-treated MS patients. Neurology 70:1150–1151
Yaldizli O, Glassl S, Sturm D, Papadopoulou A, Gass A, Tettenborn B, Putzki N (2011) Fatigue and progression of corpus callosum atrophy in multiple sclerosis. J Neurol 258:2199–2205
Yousry TA, Major EO, Ryschkewitsch C, Fahle G, Fischer S, Hou J, Curfman B, Miszkiel K, Mueller-Lenke N, Sanchez E, Barkhof F, Radue EW, Jager HR, Clifford DB (2006) Evaluation of patients treated with natalizumab for progressive multifocal leukoencephalopathy. N Engl J Med 354:924–933
Yousry TA, Pelletier D, Cadavid D, Gass A, Richert ND, Radue EW, Filippi M (2012) MRI pattern in natalizumab-associated progressive multifocal leukoencephalopathy. Ann Neurol. doi:10.1002/ana.23676
Conflicts of interest
The authors declare that they have no conflict of interest related to the publication of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rocca, M.A., Messina, R. & Filippi, M. Multiple sclerosis imaging: recent advances. J Neurol 260, 929–935 (2013). https://doi.org/10.1007/s00415-012-6788-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-012-6788-8