Abstract
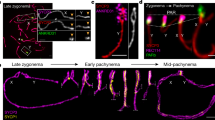
Developmental progress of germ cells through meiotic phases is closely tied to ongoing meiotic recombination. In mammals, recombination preferentially occurs in genomic regions known as hotspots; the protein that activates these hotspots is PRDM9, containing a genetically variable zinc finger (ZNF) domain and a PR-SET domain with histone H3K4 trimethyltransferase activity. PRDM9 is required for fertility in mice, but little is known about its localization and developmental dynamics. Application of spermatogenic stage-specific markers demonstrates that PRDM9 accumulates in male germ cell nuclei at pre-leptonema to early leptonema but is no longer detectable in nuclei by late zygonema. By the pachytene stage, PRDM9-dependent histone H3K4 trimethyl marks on hotspots also disappear. PRDM9 localizes to nuclei concurrently with the deposition of meiotic cohesin complexes, but is not required for incorporation of cohesin complex proteins into chromosomal axial elements, or accumulation of normal numbers of RAD51 foci on meiotic chromatin by late zygonema. Germ cells lacking PRDM9 exhibit inefficient homology recognition and synapsis, with aberrant repair of meiotic DNA double-strand breaks and transcriptional abnormalities characteristic of meiotic silencing of unsynapsed chromatin. Together, these results on the developmental time course for nuclear localization of PRDM9 establish its direct window of function and demonstrate the independence of chromosome axial element formation from the concurrent PRDM9-mediated activation of recombination hotspots.










Similar content being viewed by others
References
Anderson EL, Baltus AE, Roepers-Gajadien HL, Hassold TJ, de Rooij DG, van Pelt AM, Page DC (2008) Stra8 and its inducer, retinoic acid, regulate meiotic initiation in both spermatogenesis and oogenesis in mice. Proc Natl Acad Sci U S A 105:14976–14980
Ashley T, Plug AW, Xu JH, Solari AJ, Reddy G, Golub EI, Ward DC (1995) Dynamic changes in Rad51 distribution on chromatin during meiosis in male and female vertebrates. Chromosoma 104:19–28
Baarends WM, Wassenaar E, van der Laan R, Hoogerbrugge J, Sleddens-Linkels E, Hoeijmakers HJ, de Boer P, Grootegoed JA (2005) Silencing of unpaired chromatin and histone H2A ubiquitination in mammalian meiosis. Mol Cell Biol 25:1041–1053
Baker CL, Walker M, Kajita S, Petkov PM, Paigen K (2014) PRDM9 binding organizes hotspot nucleosomes and limits Holliday junction migration. Genome Res 24:724–732
Bannister L, Pezza R, Donaldson J, de Rooij DG, Schimenti K, Camerini-Otero RD, Schimenti JC (2007) Male-specific sterility in mice carrying a dominant, recombination-defective allele of the RecA homolog Dmc1. PLoS Biol 5:e105
Barchi M, Mahadevaiah S, Di Giacomo M, Baudat F, de Rooij DG, Burgoyne PS, Jasin M, Keeney S (2005) Surveillance of different recombination defects in mouse spermatocytes yields distinct responses despite elimination at an identical developmental stage. Mol Cell Biol 25:7203–7215
Baudat F, Manova K, Yuen JP, Jasin M, Keeney S (2000) Chromosome synapsis defects and sexually dimorphic meiotic progression in mice lacking Spo11. Mol Cell 6:989–998
Baudat F, Buard J, Fledel-Alon A, Ober C, Przeworski M, Coop G, de Massy B (2010) PRDM9 is a major determinant of meiotic recombination hotspots in humans and mice. Science 327:836–840
Baudat F, Imai Y, de Massy B (2013) Meiotic recombination in mammals: localization and regulation. Nat Rev Genet 14:794–806
Bhattacharyya T, Gregorova S, Mihola O, Anger M, Sebestova J, Denny P, Simecek P, Forejt J (2013) Mechanistic basis of infertility of mouse intersubspecific hybrids. Proc Natl Acad Sci U S A 110:E468–E477
Bhattacharyya T, Reifova R, Gregorova S, Simecek P, Gergelits V, Mistrik M, Martincova I, Pialek J, Forejt J (2014) X chromosome control of meiotic chromosome synapsis in mouse inter-subspecific hybrids. PLoS Genet 10:e1004088
Billings T, Parvanov ED, Baker CL, Walker M, Paigen K, Petkov PM (2013) DNA binding specificities of the long zinc-finger recombination protein PRDM9. Genome Biol 14:R35
Boateng KA, Bellani MA, Gregoretti IV, Pratto F, Camerini-Otero RD (2013) Homologous pairing preceding SPO11-mediated double-strand breaks in mice. Dev Cell 24:196–205
Bolcun-Filas E, Schimenti JC (2012) Genetics of meiosis and recombination in mice. Int Rev Cell Mol Biol 298:179–227
Borde V, de Massy B (2013) Programmed induction of DNA double strand breaks during meiosis: setting up communication between DNA and the chromosome structure. Curr Opin Genet Dev 23:147–155
Brick K, Smagulova F, Khil P, Camerini-Otero RD, Petukhova GV (2012) Genetic recombination is directed away from functional genomic elements in mice. Nature 485:642–645
Buaas FW, Kirsh AL, Sharma M, McLean DJ, Morris JL, Griswold MD, de Rooij DG, Braun RE (2004) Plzf is required in adult male germ cells for stem cell self-renewal. Nat Genet 36:647–652
Burgoyne PS, Mahadevaiah SK, Turner JM (2009) The consequences of asynapsis for mammalian meiosis. Nat Rev Genet 10:207–216
Bustin SA (2000) Absolute quantification of mRNA using real-time reverse transcription polymerase chain reaction assays. J Mol Endocrinol 25:169–193
Cobb J, Reddy RK, Park C, Handel MA (1997) Analysis of expression and function of topoisomerase I and II during meiosis in male mice. Mol Reprod Dev 46:489–498
Cobb J, Cargile B, Handel MA (1999) Acquisition of competence to condense metaphase I chromosomes during spermatogenesis. Dev Biol 205:49–64
Cohen PE, Pollack SE, Pollard JW (2006) Genetic analysis of chromosome pairing, recombination, and cell cycle control during first meiotic prophase in mammals. Endocr Rev 27:398–426
Daniel K, Lange J, Hached K, Fu J, Anastassiadis K, Roig I, Cooke HJ, Stewart AF, Wassmann K, Jasin M, Keeney S, Toth A (2011) Meiotic homologue alignment and its quality surveillance are controlled by mouse HORMAD1. Nat Cell Biol 13:599–610
de Massy B (2013) Initiation of meiotic recombination: how and where? Conservation and specificities among eukaryotes. Annu Rev Genet 47:563–599
de Vries FA, de Boer E, van den Bosch M, Baarends WM, Ooms M, Yuan L, Liu JG, van Zeeland AA, Heyting C, Pastink A (2005) Mouse Sycp1 functions in synaptonemal complex assembly, meiotic recombination, and XY body formation. Genes Dev 19:1376–1389
Eaker S, Pyle A, Cobb J, Handel MA (2001) Evidence for meiotic spindle checkpoint from analysis of spermatocytes from Robertsonian-chromosome heterozygous mice. J Cell Sci 114:2953–2965
Eijpe M, Offenberg H, Jessberger R, Revenkova E, Heyting C (2003) Meiotic cohesin REC8 marks the axial elements of rat synaptonemal complexes before cohesins SMC1 beta and SMC3. J Cell Biol 160:657–670
Fujiwara Y, Ogonuki N, Inoue K, Ogura A, Handel MA, Noguchi J, Kunieda T (2013) t-SNARE Syntaxin2 (STX2) is implicated in intracellular transport of sulfoglycolipids during meiotic prophase in mouse spermatogenesis. Biol Reprod 88:141
Fukuda T, Fukuda N, Agostinho A, Hernandez-Hernandez A, Kouznetsova A, Hoog C (2014) STAG3-mediated stabilization of REC8 cohesin complexes promotes chromosome synapsis during meiosis. EMBO J 33:1243–1255
Gutierrez-Caballero C, Herran Y, Sanchez-Martin M, Suja JA, Barbero JL, Llano E, Pendas AM (2011) Identification and molecular characterization of the mammalian alpha-kleisin RAD21L. Cell Cycle 10:1477–1487
Handel MA, Schimenti JC (2010) Genetics of mammalian meiosis: regulation, dynamics and impact on fertility. Nat Rev Genet 11:124–136
Handel MA, Lessard C, Reinholdt L, Schimenti J, Eppig JJ (2006) Mutagenesis as an unbiased approach to identify novel contraceptive targets. Mol Cell Endocrinol 250:201–205
Hayashi K, Matsui Y (2006) Meisetz, a novel histone tri-methyltransferase, regulates meiosis-specific epigenesis. Cell Cycle 5:615–620
Hayashi K, Yoshida K, Matsui Y (2005) A histone H3 methyltransferase controls epigenetic events required for meiotic prophase. Nature 438:374–378
Herran Y, Gutierrez-Caballero C, Sanchez-Martin M, Hernandez T, Viera A, Barbero JL, de Alava E, de Rooij DG, Suja JA, Llano E, Pendas AM (2011) The cohesin subunit RAD21L functions in meiotic synapsis and exhibits sexual dimorphism in fertility. EMBO J 30:3091–3105
Hopkins J, Hwang G, Jacob J, Sapp N, Bedigian R, Oka K, Overbeek P, Murray S, Jordan PW (2014) Meiosis-specific cohesin component, Stag3 is essential for maintaining centromere chromatid cohesion, and required for DNA repair and synapsis between homologous chromosomes. PLoS Genet 10:e1004413
Ichijima Y, Ichijima M, Lou Z, Nussenzweig A, Camerini-Otero RD, Chen J, Andreassen PR, Namekawa SH (2011) MDC1 directs chromosome-wide silencing of the sex chromosomes in male germ cells. Genes Dev 25:959–971
Ishiguro K, Kim J, Fujiyama-Nakamura S, Kato S, Watanabe Y (2011) A new meiosis-specific cohesin complex implicated in the cohesin code for homologous pairing. EMBO Rep 12:267–275
Jin H, Guacci V, Yu HG (2009) Pds5 is required for homologue pairing and inhibits synapsis of sister chromatids during yeast meiosis. J Cell Biol 186:713–725
Kauppi L, Barchi M, Lange J, Baudat F, Jasin M, Keeney S (2013) Numerical constraints and feedback control of double-strand breaks in mouse meiosis. Genes Dev 27:873–886
Kotaja N, Kimmins S, Brancorsini S, Hentsch D, Vonesch J-L, Davidson I, Parvinen M, Sassone-Corsi P (2004) Preparation, isolation and characterization of stage-specific spermatogenic cells for cellular and molecular analysis. Nat Methods 1:249–254
Koubova J, Hu YC, Bhattacharyya T, Soh YQ, Gill ME, Goodheart ML, Hogarth CA, Griswold MD, Page DC (2014) Retinoic acid activates two pathways required for meiosis in mice. PLoS Genet 10:e1004541
La Salle S, Sun F, Handel MA (2009) Isolation and short-term culture of mouse spermatocytes for analysis of meiosis. In: Keeney S (ed) Methods in molecular biology, molecular medicine and biotechnology: Meiosis Protocols Humana Press, pp 279–297
Llano E, Herran Y, Garcia-Tunon I, Gutierrez-Caballero C, de Alava E, Barbero JL, Schimenti J, de Rooij DG, Sanchez-Martin M, Pendas AM (2012) Meiotic cohesin complexes are essential for the formation of the axial element in mice. J Cell Biol 197:877–885
Llano E, Gomez HL, Garcia-Tunon I, Sanchez-Martin M, Caburet S, Barbero JL, Schimenti JC, Veitia RA, Pendas AM (2014) STAG3 is a strong candidate gene for male infertility. Hum Mol Genet 23:3421–3431
Mahadevaiah SK, Turner JMA, Baudat F, Rogakou EP, de Boer P, Blanco-Rodriguez J, Jasin M, Keeney S, Bonner WM, Burgoyne PS (2001) Recombinational DNA double-strand breaks in mice precede synapsis. Nat Genet 27:271–276
Major AT, Whiley PA, Loveland KL (2011) Expression of nucleocytoplasmic transport machinery: clues to regulation of spermatogenic development. Biochim Biophys Acta 1813:1668–1688
Novak I, Wang H, Revenkova E, Jessberger R, Scherthan H, Hoog C (2008) Cohesin Smc1beta determines meiotic chromatin axis loop organization. J Cell Biol 180:83–90
Paigen K, Petkov P (2010) Mammalian recombination hot spots: properties, control and evolution. Nat Rev Genet 11:221–233
Parvanov ED, Petkov PM, Paigen K (2010) Prdm9 controls activation of mammalian recombination hotspots. Science 327:835
Parvinen M, Toppari J, Lahdetie J (1993) Transillumination phase contrast microscope techniques for evaluation of male germ cell toxicity and mutagenicity. In: Heindel JJ (ed) Methods toxicol. Academic, San Diego, pp 142–165
Peters AHFM, Plug AW, van Vugt MJ, de Boer P (1997) A drying-down technique for the spreading of mammalian meiocytes from the male and female germline. Chromosom Res 5:66–71
Pezzi N, Prieto I, Kremer L, Jurado LAP, Valero C, Del Mazo J, Martinez C, Barbero JL (2000) STAG3, a novel gene encoding a protein involved in meiotic chromosome pairing and location of STAG3-related genes flanking the Williams-Beuren syndrome deletion. FASEB J 14:581–592
Pittman DL, Cobb J, Schimenti KJ, Wilson LA, Cooper DM, Brignull E, Handel MA, Schimenti JC (1998) Meiotic prophase arrest with failure of chromosome synapsis in mice deficient for Dmc1, a germline-specific RecA homolog. Mol Cell 1:697–705
Prieto I, Suja JA, Pezzi N, Kremer L, Martinez C, Rufas JS, Barbero JL (2001) Mammalian STAG3 is a cohesin specific to sister chromatid arms in meiosis I. Nat Cell Biol 3:761–766
Revenkova E, Jessberger R (2006) Shaping meiotic prophase chromosomes: cohesins and synaptonemal complex proteins. Chromosoma 115:235–240
Revenkova E, Eijpe M, Heyting C, Gross B, Jessberger R (2001) Novel meiosis-specific isoform of mammalian SMC1. Mol Cell Biol 21:6984–6998
Rogers RS, Inselman A, Handel MA, Matunis MJ (2004) SUMO modified proteins localize to the XY body of pachytene spermatocytes. Chromosoma 113:233–243
Romanienko PJ, Camerini-Otero RD (2000) The mouse Spo11 gene is required for meiotic chromosome synapsis. Mol Cell 6:975–987
Rosen B, Beddington RS (1993) Whole-mount in situ hybridization in the mouse embryo: gene expression in three dimensions. Trends Genet 9:162–167
Scherthan H (2001) A bouquet makes ends meet. Nat Rev Mol Cell Biol 2:621–627
Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez JY, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A (2012) Fiji: an open-source platform for biological-image analysis. Nat Methods 9:676–682
Sun F, Handel MA (2008) Regulation of the meiotic prophase I to metaphase I transition in mouse spermatocytes. Chromosoma 117:471–485
Turner JM (2007) Meiotic sex chromosome inactivation. Development 134:1823–1831
Turner JMA, Mahadevaiah SK, Fernandez-Capetillo O, Nussenzweig A, Xu X, Deng C-X, Burgoyne PS (2005) Silencing of unsynapsed meiotic chromosomes in the mouse. Nat Genet 37:41–47
Vrooman LA, Oatley JM, Griswold JE, Hassold TJ, Hunt PA (2015) Estrogenic exposure alters the spermatogonial stem cells in the developing testis, permanently reducing crossover levels in the adult. PLoS Genet 11:e1004949
Weiss J, Hurley LA, Harris RM, Finlayson C, Tong M, Fisher LA, Moran JL, Beier DR, Mason C, Jameson JL (2012) ENU mutagenesis in mice identifies candidate genes for hypogonadism. Mamm Genome 23:346–355
Xu H, Beasley MD, Warren WD, van der Horst GT, McKay MJ (2005) Absence of mouse REC8 cohesin promotes synapsis of sister chromatids in meiosis. Dev Cell 8:949–961
Yoshinaga K, Nishikawa S, Ogawa M, Hayashi S-I, Kunisada T, Fujimoto T, Nishikawa S-I (1991) Role of c-kit in mouse spermatogenesis: identification of spermatogonia as a specific site of c-kit expression and function. Development 113:689–699
Zhou Q, Li Y, Nie R, Friel P, Mitchell D, Evanoff RM, Pouchnik DJ, Banasik B, McCarrey JR, Small C, Griswold MD (2008) Expression of Stimulated by retinoic acid gene 8 (Stra8) and maturation of murine gonocytes and spermatogonia induced by retinoic acid in vitro. Biol Reprod 78:537–545
Acknowledgments
We are indebted to members of the Handel and Paigen laboratories for discussion of this work in progress, to Kristina Palmer and Sabrina Petri for animal care and technical assistance, to Dr. G. Carter for statistical consultation, and to Drs. G. Carter, J. Eppig and S. Handel for critical comments on the manuscript. We are grateful to Y. Watanabe and S. Namekawa for providing antibodies. This work was supported by P01 grants from the NIH (HD42137 and GM99640) and by fellowships to YF from the Japan Society for the Promotion of Science (JSPS) and the Strategic Young Researcher Oversea Visits Program for Accelerating Brain Research. Research reported in this publication was also partially supported by the National Cancer Institute under award number P30 CA034196; the content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig. 1
Expression of Prdm9 and nuclear localization of PRDM9 protein. a qRT-PCR showing the developmental pattern of Prdm9 transcript expression during the juvenile onset of spermatogenesis. b Western blot showing developmental pattern of expression of PRDM9 protein in testis during the juvenile onset of spermatogenesis. TUBA1A is the loading control. c Nuclear localization of PRDM9 was visualized in HEK293 cells transfected with FLAG-tagged Prdm9 after immunolabeling with pre-immune serum (left panel), with anti-PRDM9 antibody in green (middle panel), and with anti-FLAG antibody in green (right panel). DNA was counterstained with DAPI (blue). The co-localization of PRDM9 with nuclear DAPI signal suggests inherent nuclear localization of PRDM9 protein, even in a heterologous system. Scale bar = 20 μm (GIF 112 kb)
Supplementary Fig. 2
PRDM9 in the nuclei of germ cells during early meiotic prophase I. a, b PRDM9 in germ cells at early meiosis I prophase is revealed by immunolabeling of fibrin clot-embedded germ cells with a combination of anti-SYCP3 (red) to mark the SC and anti-PRDM9 (green). PRDM9 was not detected in pachytene spermatocytes (arrows in a) but was detected in germ cells with the patchy SYCP3 labeling typical of pre-leptotene/leptotene spermatocytes (arrows in b). c–f PRDM9 is detected in the nuclei of spermatocytes co-labeled with EdU during the pre-meiotic S-phase. Juvenile males (8 dpp) were injected with EdU for a 6-h pulse, followed by removal of testes for immunolabeling. Whole-mounted seminiferous tubules were immunolabeled with PRDM9 antibody (green); the incorporation of EdU was detected with Alexa Fluor®594 (red); and nuclei were counterstained with DAPI (blue in c and f). Localization of PRDM9 in EdU-positive germ cells provides further evidence that PRDM9 is initially expressed in S-phase/post-S-phase pre-leptotene/leptotene spermatocytes (arrow in e–f). Scale bars = 20 μm (GIF 113 kb)
Supplementary Fig. 3
Abnormal silencing of unsynapsed chromatin in Prdm9-deficient pachytene-like spermatocytes. Spread chromatin preparations from wild-type (a) and Prdm9 M1045Lja-mutant (b) spermatocytes were labeled with antibodies recognizing SUMO1 (green), a marker of transcriptional silencing, SYCP3 (blue) to mark the SC, P-H2AFX (red). The co-localization of SUMO1 and P-H2AFX (arrows in b) provides evidence for transcriptional silencing. Scale bar = 20 μm (GIF 29 kb)
Rights and permissions
About this article
Cite this article
Sun, F., Fujiwara, Y., Reinholdt, L.G. et al. Nuclear localization of PRDM9 and its role in meiotic chromatin modifications and homologous synapsis. Chromosoma 124, 397–415 (2015). https://doi.org/10.1007/s00412-015-0511-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00412-015-0511-3