Abstract
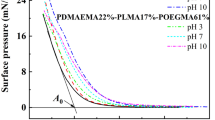
Aggregation behavior of three star-shaped fluoropolymers containing polyhedral oligomeric silsesquioxane (POSS) and different monomers poly(methyl methacrylate) (PMMA), poly(trifluoroethyl methacrylate) (PTFEMA) and poly[poly(ethylene glycol)methyl ether methacrylate] (PMPEGMA), POSS-(PMMA-b-PTFEMA)8, POSS-(PTFEMA-b-PMMA)8, and POSS-(PTFEMA-b-PMPEGMA)8) was investigated at the air–water interface. The interfacial rheology and surface morphology were studied through surface pressure and compression modulus measurements, hysteresis, relaxation, atomic force microscopy (AFM), and X-ray photoelectron spectroscopy (XPS). The surface pressure–mean molecular area (Π-MMA) isotherms exhibited four regions, and it was found that the pseudoplateau observed corresponds to a “pancake-to-brush” transition. The compression modulus isotherms showed two peak values which were related to the phase transition of the monolayer. The hysteresis behaviors were compared in two different regions during the compression–expansion process. In addition, the relaxation of the monolayer was discussed using the step compression method. The relaxation process was proposed that related to the adsorption–desorption exchange of molecules and polymer segments on the surface (fast relaxation process) and the reconformation of the adsorbed macromolecules inside the adsorption layer (slow relaxation process). Furthermore, the Langmuir–Blodgett (LB) films prepared at different surface pressures from the three copolymers were scanned by AFM and a variety of morphologies were observed. Finally, the structure evolutions for the three star-shaped copolymers that aggregate at the air–water interface were proposed.










Similar content being viewed by others
References
Brzozowska A, Paczesny J, Parzuchowski P, Kusznerczuk M, Nikiforov K, Rokicki G, Gregorowicz J (2014) Hyperbranched polyesters termienated with alkyl chains of different length at the air/water interface and on solid substrates. Macromolecules 47(15):5256–5268. doi:10.1021/Ma500941c
Kim H-J, Deng J, Hoyt Lalli J, Riffle JS, Viers BD, Esker AR (2005) Blends of amphiphilic trisilanolisobutyl-POSS and phosphine oxide substituted poly(dimethylsiloxane) at the air/water interface. Langmuir 21(5):1908–1916. doi:10.1021/la0475674
Felipe MJ, Estillore N, Pernites RB, Nguyen T, Ponnapati R, Advincula RC (2011) Interfacial behavior of OEG–linear dendron monolayers: aggregation, nanostructuring, and electropolymerizability. Langmuir 27(15):9327–9336. doi:10.1021/la200916n
Lenis J, Razavi S, Cao KD, Lin B, Lee KYC, Tu RS, Kretzschmar I (2015) Mechanical stability of polystyrene and janus particle monolayers at the air/water interface. J Am Chem Soc 137(49):15370–15373. doi:10.1021/jacs.5b10183
Kim HC, Lee H, Khetan J, Won Y-Y (2015) Surface mechanical and rheological behaviors of biocompatible poly((d,l-lactic acid-ran-glycolic acid)-block-ethylene glycol) (PLGA–PEG) and poly((d,l-lactic acid-ran-glycolic acid-ran-ε-caprolactone)-block-ethylene glycol) (PLGACL–PEG) block copolymers at the air–water interface. Langmuir 31(51):13821–13833. doi:10.1021/acs.langmuir.5b03622
Claro PCD, Coustet ME, Diaz C, Maza E, Cortizo MS, Requejo FG, Pietrasanta LI, Ceolin M, Azzaroni O (2013) Self-assembly of PBzMA-b-PDMAEMA diblock copolymer films at the air-water interface and deposition on solid substrates via Langmuir-Blodgett transfer. Soft Matter 9(45):10899–10912. doi:10.1039/c3sm52336e
Joncheray TJ, Bernard SA, Matmour R, Lepoittevin B, El-Khouri RJ, Taton D, Gnanou Y, Duran RS (2007) Polystyrene-b-poly(tert-butyl acrylate) and polystyrene-b-poly(acrylic acid) dendrimer-like copolymers: two-dimensional self-assembly at the air−water interface. Langmuir 23(5):2531–2538. doi:10.1021/la062924r
Cheyne RB, Moffitt MG (2006) Self-assembly of polystyrene-block-poly(ethylene oxide) copolymers at the air−water interface: is dewetting the genesis of surface aggregate formation? Langmuir 22(20):8387–8396. doi:10.1021/la061953z
Logan JL, Masse P, Gnanou Y, Taton D, Duran RS (2005) Polystyrene-block-poly(ethylene oxide) stars as surface films at the air/water interface. Langmuir 21(16):7380–7389. doi:10.1021/la050787c
Li Z, Ma X, Zang D, Guan X, Zhu L, Liu J, Chen F (2015) Interfacial rheology and aggregation behaviour of amphiphilic CBABC-type pentablock copolymers at the air-water interface: effects of block ratio and chain length. RSC Adv 5(101):82869–82878. doi:10.1039/c5ra08109b
Wang X, Ma X, Zang D (2013) Aggregation behavior of polystyrene-b-poly(acrylic acid) at the air-water interface. Soft Matter 9(2):443–453
Perepichka II, Borozenko K, Badia A, Bazuin CG (2011) Pressure-induced order transition in nanodot-forming diblock copolymers at the air/water interface. J Am Chem Soc 133(49):19702–19705. doi:10.1021/ja209502d
Kraska M, Gallei M, Stühn B, Rehahn M (2013) Pressure induced structure formation in Langmuir monolayers of amphiphilic metallocene diblock copolymers. Langmuir 29(26):8284–8291
Chen YJ, Liu T, Xu GY, Zhang J, Zhai XR, Yuan J, Tan YB (2015) Aggregation behavior of X-shaped branched block copolymers at the air/water interface: effect of block sequence and temperature. Colloid Polym Sci 293(1):97–107. doi:10.1007/s00396-014-3392-8
Naolou T, Busse K, Lechner BD, Kressler J (2014) The behavior of poly(-caprolactone) and poly(ethylene oxide)-b-poly(-caprolactone) grafted to a poly(glycerol adipate) backbone at the air/water interface. Colloid Polym Sci 292(5):1199–1208. doi:10.1007/s00396-014-3168-1
Stanimirova RD, Gurkov TD, Kralchevsky PA, Balashev KT, Stoyanov SD, Pelan EG (2013) Surface pressure and elasticity of hydrophobin HFBII layers on the air–water interface: rheology versus structure detected by AFM imaging. Langmuir 29(20):6053–6067
Li Destri G, Miano F, Marletta G (2014) Structure–rheology relationship in weakly amphiphilic block copolymer Langmuir monolayers. Langmuir 30(12):3345–3353. doi:10.1021/la4043777
Wamke A, Dopierała K, Prochaska K, Maciejewski H, Biadasz A, Dudkowiak A (2015) Characterization of Langmuir monolayer, Langmuir–Blodgett and Langmuir–Schaefer films formed by POSS compounds. Colloids Surf A Physicochem Eng Asp 464(0):110–120. doi:10.1016/j.colsurfa.2014.10.022
Dopierala K, Wamke A, Dutkiewicz M, Maciejewski H, Prochaska K (2014) Interfacial properties of fully condensed functional silsesquioxane: a Langmuir monolayer study. J Phys Chem C 118(42):24548–24555. doi:10.1021/jp507670c
Kucuk AC, Matsui J, Miyashita T (2011) Langmuir-Blodgett films composed of amphiphilic double-decker shaped polyhedral oligomeric silsesquioxanes. J Colloid Interface Sci 355(1):106–114. doi:10.1016/j.jcis.2010.12.033
Deng J, Polidan JT, Hottle JR, Farmer-Creely CE, Viers BD, Esker AR (2002) Polyhedral oligomeric silsesquioxanes: a new class of amphiphiles at the air/water interface. J Am Chem Soc 124(51):15194–15195. doi:10.1021/ja027860v
Hottle JR, Deng J, Kim H-J, Farmer-Creely CE, Viers BD, Esker AR (2005) Blends of amphiphilic poly(dimethylsiloxane) and nonamphiphilic octaisobutyl-POSS at the air/water interface. Langmuir 21(6):2250–2259. doi:10.1021/la047565j
Yin W, Deng J, Esker AR (2009) Surface rheology of trisilanolisobutyl−POSS at the air/water interface. Langmuir 25(13):7181–7184. doi:10.1021/la900397r
Mitsuishi M, Zhao F, Kim Y, Watanabe A, Miyashita T (2008) Preparation of ultrathin silsesquioxane nanofilms via polymer Langmuir−Blodgett films. Chem Mater 20(13):4310–4316. doi:10.1021/cm800067j
Lee W, Ni S, Deng J, Kim B-S, Satija SK, Mather PT, Esker AR (2007) Telechelic poly(ethylene glycol)−POSS amphiphiles at the air/water interface. Macromolecules 40(3):682–688. doi:10.1021/ma0618171
Maestro A, Ortega F, Monroy F, Krägel J, Miller R (2009) Molecular weight dependence of the shear rheology of poly(methyl methacrylate) Langmuir films: a comparison between two different rheometry techniques. Langmuir 25(13):7393–7400. doi:10.1021/la9003033
Seo Y, Cho CY, Hwangbo M, Choi HJ, Hong SM (2008) Effect of temperature on the interfacial behavior of a polystyrene-b-poly(methyl methacrylate) diblock copolymer at the air/water interface. Langmuir 24(6):2381–2386. doi:10.1021/la702745w
Maestro A, Ortega F, Rubio RG, Rubio MA, Krägel J, Miller R (2011) Rheology of poly(methyl methacrylate) Langmuir monolayers: percolation transition to a soft glasslike system. J Chem Phys 134(10):104704. doi:10.1063/1.3560612
Wang Z, Wen G, Zhao F, Huang C, Wang X, Shi T, Li H (2014) Effect of selective solvent on the aggregate behavior of the mixed Langmuir monolayers of PS-b-PEO and PS-b-PMMA. RSC Adv 4(56):29595–29603. doi:10.1039/c4ra04161e
Miñones J, Conde MMO, Yebra-Pimentel E, Trillo JM (2009) Behavior of syndiotactic poly(methyl methacrylate) monolayers at the air/water interface: influence of temperature and molecular weight on the surface pressure−area isotherms and Brewster angle microscopy images. J Phys Chem C 113(40):17455–17463. doi:10.1021/jp904848c
Fuchs C, Hussain H, Schwieger C, Schulz M, Binder WH, Kressler J (2015) Molecular arrangement of symmetric and non-symmetric triblock copolymers of poly(ethylene oxide) and poly(isobutylene) at the air/water interface. J Colloid Interface Sci 437:80–89. doi:10.1016/j.jcis.2014.09.050
Park H-W, Choi J, Ohn K, Lee H, Kim JW, Won Y-Y (2012) Study of the air–water interfacial properties of biodegradable polyesters and their block copolymers with poly(ethylene glycol). Langmuir 28(31):11555–11566. doi:10.1021/la300810q
Lopes SI, Gonçalves da Silva AM, Brogueira P, Piçarra S, Martinho J (2007) Interfacial behavior of poly (isoprene-b-methyl methacrylate) diblock copolymers and their blends with polystyrene at the air-water interface. Langmuir 23(18):9310–9319
Matmour R, Francis R, Duran RS, Gnanou Y (2005) Interfacial behavior of anionically synthesized amphiphilic star block copolymers based on polybutadiene and poly(ethylene oxide) at the air/water interface. Macromolecules 38(18):7754–7767. doi:10.1021/ma050578z
Tong LJ, Bao MT, Li YM, Gong HY (2014) Interfacial dynamic and dilational rheology of polyelectrolyte/surfactant two-component nanoparticle systems at air–water interface. Appl Surf Sci 316(0):147–154. doi:10.1016/j.apsusc.2014.07.186
Gzyl-Malcher B, Filek M, Brezesinski G (2011) Mixed DPPC/DPTAP monolayers at the air/water interface: influence of indolilo-3-acetic acid and selenate ions on the monolayer morphology. Langmuir 27(17):10886–10893. doi:10.1021/la201765u
Conde MM, Conde O, Trillo JM, Miñones J (2011) Approach to knowledge of the interaction between the constituents of contact lenses and ocular tears: mixed monolayers of poly(methyl methacrylate) and dipalmitoyl phosphatidyl choline. Langmuir 27(7):3424–3435. doi:10.1021/la1051172
Spigone E, Cho G-Y, Fuller GG, Cicuta P (2009) Surface rheology of a polymer monolayer: effects of polymer chain length and compression rate. Langmuir 25(13):7457–7464. doi:10.1021/la900385y
Martín-García B, Velázquez MM (2013) Block copolymer assisted self-assembly of nanoparticles into Langmuir–Blodgett films: effect of polymer concentration. Mater Chem Phys 141(1):324–332
Zhu H, Matsui J, Yamamoto S, Miyashita T, Mitsuishi M (2015) Solvent-dependent properties of poly(vinylidene fluoride) monolayers at the air-water interface. Soft Matter 11(10):1962–1972. doi:10.1039/C4sm02800g
Martín-García B, Velázquez MM, Pérez-Hernández JA, Hernández-Toro J (2010) Langmuir and Langmuir−Blodgett films of a maleic anhydride derivative: effect of subphase divalent cations. Langmuir 26(18):14556–14562. doi:10.1021/la101736e
Hilles HM, Ortega F, Rubio RG, Monroy F (2004) Long-time relaxation dynamics of Langmuir films of a glass-forming polymer: evidence of glasslike dynamics in two dimensions. Phys Rev Lett 92(25):255503
Gargallo L, Becerra N, Encinas MV, Ortega F, Rubio RG, Leiva A, Radic D (2015) Amphiphilic 2-ethyl hexyl methacrylate-b-N,N ’-dimethylacrylamide diblock copolymer monolayer behaviour at the air-water interface. Polym Int 64(6):740–749. doi:10.1002/Pi.4845
Klein CO, de Viguerie L, Christopoulou C, Jonas U, Clark CG, Müllen K, Vlassopoulos D (2011) Viscoelasticity of semifluorinated alkanes at the air/water interface. Soft Matter 7(17):7737–7746. doi:10.1039/c1sm05357d
Njikang GN, Cao L, Gauthier M (2008) Pressure- and temperature-induced association of arborescent polystyrene-graft-poly(ethylene oxide) copolymers at the air−water interface. Langmuir 24(22):12919–12927. doi:10.1021/la802163k
Juárez J, Goy-López S, Cambón A, Valdez MA, Taboada P, Vc M (2010) Surface properties of monolayers of amphiphilic poly (ethylene oxide)-poly (styrene oxide) block copolymers. J Phys Chem C 114(37):15703–15712
Glagola CP, Miceli LM, Milchak MA, Halle EH, Logan JL (2012) Polystyrene–poly(ethylene oxide) diblock copolymer: the effect of polystyrene and spreading concentration at the air/water interface. Langmuir 28(11):5048–5058. doi:10.1021/la204100d
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Funding
This study was funded by the National Natural Science Foundation of China (Grant No. 51372206), Natural Science Foundation of Shaanxi Province (Grant No. 2013JM2012), and NPU Foundation for Fundamental Research (3102014JCQ01089).
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
ESM 1
(DOC 6851 kb)
Rights and permissions
About this article
Cite this article
Li, Z., Ma, X., Guan, X. et al. Aggregation behavior of star-shaped fluoropolymers containing polyhedral oligomeric silsesquioxane (POSS) at the air–water interface. Colloid Polym Sci 295, 157–170 (2017). https://doi.org/10.1007/s00396-016-3986-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-016-3986-4