Abstract
Key message
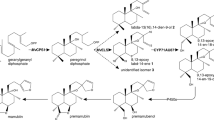
CYP716A179, a cytochrome P450 monooxygenase expressed predominantly in tissue-cultured stolons of licorice ( Glycyrrhiza uralensis ), functions as a triterpene C-28 oxidase in the biosynthesis of oleanolic acid and betulinic acid.
Abstract
Cytochrome P450 monooxygenases (P450s) play key roles in the structural diversification of plant triterpenoids. Among these, the CYP716A subfamily, which functions mainly as a triterpene C-28 oxidase, is common in plants. Licorice (Glycyrrhiza uralensis) produces bioactive triterpenoids, such as glycyrrhizin and soyasaponins, and relevant P450s (CYP88D6, CYP72A154, and CYP93E3) have been identified; however, no CYP716A subfamily P450 has been isolated. Here, we identify CYP716A179, which functions as a triterpene C-28 oxidase, by RNA sequencing analysis of tissue-cultured stolons of G. uralensis. Heterologous expression of CYP716A179 in engineered yeast strains confirmed the production of oleanolic acid, ursolic acid, and betulinic acid from β-amyrin, α-amyrin, and lupeol, respectively. The transcript level of CYP716A179 was about 500 times higher in tissue-cultured stolons than in intact roots. Oleanolic acid and betulinic acid were consistently detected only in tissue-cultured stolons. The discovery of CYP716A179 helps increase our understanding of the mechanisms of tissue-type-dependent triterpenoid metabolism in licorice and provides an additional target gene for pathway engineering to increase the production of glycyrrhizin in licorice tissue cultures by disrupting competing pathways.




Similar content being viewed by others
Abbreviations
- aAS:
-
α-Amyrin synthase
- bAS:
-
β-Amyrin synthase
- CAS:
-
Cycloartenol synthase
- CDS:
-
Coding sequence
- CPR:
-
Cytochrome P450 reductase
- EST:
-
Expressed sequence tag
- LUS:
-
Lupeol synthase
- OSC:
-
Oxidosqualene cyclase
References
Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402
Bolger AM, Lohse M, Usadel B (2014) Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30:2114–2120
Carelli M, Biazzi E, Panara F, Tava A, Scaramelli L, Porceddu A, Graham N, Odoardi M, Piano E, Arcioni S, May S, Scotti C, Calderini O (2011) Medicago truncatula CYP716A12 is a multifunctional oxidase involved in the biosynthesis of hemolytic saponins. Plant Cell 23:3070–3081
Fukushima EO, Seki H, Ohyama K, Ono E, Umemoto N, Mizutani M, Saito K, Muranaka T (2011) CYP716A subfamily members are multifunctional oxidases in triterpenoid biosynthesis. Plant Cell Physiol 52:2050–2061
Grabherr MG, Haas BJ, Yassour M, Levin JZ, Thompson DA, Amit I, Adiconis X, Fan L, Raychowdhury R, Zeng Q, Chen Z, Mauceli E, Hacohen N, Gnirke A, Rhind N, di Palma F, Birren BW, Nusbaum C, Lindblad-Toh K, Friedman N, Regev A (2011) Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat Biotechnol 29:644–652
Hayashi H, Sudo H (2009) Economic importance of licorice. Plant Biotechnol 26:101–104
Hayashi H, Fukui H, Tabata M (1988) Examination of triterpenoids produced by callus and cell suspension cultures of Glycyrrhiza glabra. Plant Cell Rep 7:508–511
Hayashi H, Fukui H, Tabata M (1993) Distribution pattern of saponins in different organs of Glycyrrhiza glabra. Planta Med 59:351–353
Jäger S, Trojan H, Kopp T, Laszczyk MN, Scheffler A (2009) Pentacyclic triterpene distribution in various plants—rich sources for a new group of multi-potent plant extracts. Molecules 14:2016–2031
Kojoma M, Ohyama K, Seki H, Hiraoka Y, Asazu SN, Sawa S, Sekizaki H, Yoshida S, Muranaka T (2010) In vitro proliferation and triterpenoid characteristics of licorice (Glycyrrhiza uralensis Fischer, Leguminosae) stolons. Plant Biotechnol 27:59–66
Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG (2007) Clustal W and Clustal X version 2.0. Bioinformatics 23:2947–2948
Li Y, Luo H-M, Sun C, Song J-Y, Sun Y-Z, Wu Q, Wang N, Yao H, Steinmetz A, Chen S-L (2010) EST analysis reveals putative genes involved in glycyrrhizin biosynthesis. BMC Genom 11:268
Moses T, Pollier J, Shen Q, Soetaert S, Reed J, Erffelinck M-L, Van Nieuwerburgh FCW, Vanden Bossche R, Osbourn A, Thevelein JM, Deforce D, Tang K, Goossens A (2015) OSC2 and CYP716A14v2 catalyze the biosynthesis of triterpenoids for the cuticle of aerial organs of Artemisia annua. Plant Cell 27:286–301
Nelson D, Werck-Reichhart D (2011) A P450-centric view of plant evolution. Plant J 66:194–211
Oksman-Caldentey K-M, Inzé D (2004) Plant cell factories in the post-genomic era: new ways to produce designer secondary metabolites. Trends Plant Sci 9:433–440
Ramachandra Rao S, Ravishankar GA (2002) Plant cell cultures: chemical factories of secondary metabolites. Biotechnol Adv 20:101–153
Ramilowski JA, Sawai S, Seki H, Mochida K, Yoshida T, Sakurai T, Muranaka T, Saito K, Daub CO (2013) Glycyrrhiza uralensis transcriptome landscape and study of phytochemicals. Plant Cell Physiol 54:697–710
Seki H, Ohyama K, Sawai S, Mizutani M, Ohnishi T, Sudo H, Akashi T, Aoki T, Saito K, Muranaka T (2008) Licorice β-amyrin 11-oxidase, a cytochrome P450 with a key role in the biosynthesis of the triterpene sweetener glycyrrhizin. Proc Natl Acad Sci USA 105:14204–14209
Seki H, Sawai S, Ohyama K, Mizutani M, Ohnishi T, Sudo H, Fukushima EO, Akashi T, Aoki T, Saito K, Muranaka T (2011) Triterpene functional genomics in licorice for identification of CYP72A154 involved in the biosynthesis of glycyrrhizin. Plant Cell 23:4112–4123
Seki H, Tamura K, Muranaka T (2015) P450s and UGTs: key players in the structural diversity of triterpenoid saponins. Plant Cell Physiol 56:1463–1471
Sudo H, Seki H, Sakurai N, Suzuki H, Shibata D, Toyoda A, Totoki Y, Sakaki Y, Iida O, Shibata T, Kojoma M, Muranaka T, Saito K (2009) Expressed sequence tags from rhizomes of Glycyrrhiza uralensis. Plant Biotechnol 26:105–107
Thimmappa R, Geisler K, Louveau T, O’Maille P, Osbourn A (2014) Triterpene biosynthesis in plants. Annu Rev Plant Biol 65:225–257
Yasumoto S, Fukushima EO, Seki H, Muranaka T (2016) Novel triterpene oxidizing activity of Arabidopsis thaliana CYP716A subfamily enzymes. FEBS Lett 590:533–540
Acknowledgements
We are very grateful to the Takeda Garden for Medicinal Plant Conservation, Kyoto, Japan (Takeda Pharmaceutical Company Limited) for the supply of licorice plants. We thank Dr. David R. Nelson (University of Tennessee) for the naming of CYP716A179 according to the P450 nomenclature. We also thank Mr. Tsutomu Hosouchi and Ms. Sayaka Shinpo (Kazusa DNA Research Institute) for technical support with the Illumina sequencing, Dr. Kazuto Mannen (Kazusa DNA Research Institute) and Dr. Ryosuke Sano (Nara Institute of Science and Technology) for technical advice with bioinformatics tools, Dr. Kiyoshi Ohyama (Tokyo Institute of Technology) for providing oleanolic aldehyde, ursolic aldehyde and betulinic aldehyde standards, and Dr. Ery Odette Fukushima (Osaka University) for helpful discussion on phylogenetic analysis of CYP716A subfamily enzymes. This work was supported by the “Health and Labour Sciences Research Grant” on the enhancement of “Comprehensive Medicinal Plant Database”, JSPS KAKENHI Grant Number JP26450123 and research funding from the Yamada Science Foundation to H. Seki, JSPS KAKENHI Grant Number JP15H04485 to TM, and the Doctor 21 scholarship from the Yoshida Scholarship Foundation to KT.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by F. Sato.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tamura, K., Seki, H., Suzuki, H. et al. CYP716A179 functions as a triterpene C-28 oxidase in tissue-cultured stolons of Glycyrrhiza uralensis . Plant Cell Rep 36, 437–445 (2017). https://doi.org/10.1007/s00299-016-2092-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-016-2092-x