Abstract
Key message
PtHSP17.8 was regulated by various abiotic stresses. Overexpression of PtHSP17.8 enhanced the tolerance to heat and salt stresses through maintain ROS homeostasis and cooperate with stress-related genes in Arabidopsis.
Abstract
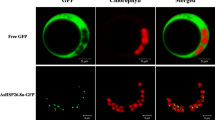
Small heat shock proteins (sHSPs) play important roles in response to diverse biotic and abiotic stresses, especially in heat tolerance. However, limited information is available on the stress tolerance roles of sHSPs in woody species. To explore the function of sHSPs in poplar, we isolated and characterized PtHSP17.8 from Populus trichocarpa. Phylogenetic analysis and subcellular localization revealed that PtHSP17.8 was a cytosolic class I sHSP. The gene expression profile of PtHSP17.8 in various tissues showed that it was significantly accumulated in stem and root, which was consistent with the GUS expression pattern driven by promoter of PtHSP17.8. The expression of PtHSP17.8 could be induced by various abiotic stresses and significantly activated by heat stress. Overexpression of PtHSP17.8 enhanced the tolerance to heat and salt stresses in Arabidopsis. The seedling survival rate, root length, relative water content, antioxidative enzyme activities, proline, and soluble sugar content were increased in transgenic Arabidopsis under heat and salt stresses, but not in normal condition. The co-expression network of PtHSP17.8 were constructed and demonstrated many stress responsive genes included. The stress-related genes in the co-expression network were up-regulated in the PtHSP17.8 overexpression seedlings. These results suggest that PtHSP17.8 confers heat and salt tolerances in plants.








Similar content being viewed by others
References
Ahuja I, de Vos RC, Bones AM, Hall RD (2010) Plant molecular stress responses face climate change. Trends Plant Sci 15:664–674
Al-Whaibi MH (2011) Plant heat-shock proteins: a mini review. J King Saud Univ Sci 23:139–150
Beauchamp C, Fridovich I (1971) Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem 44:276–287
Beers RF, Sizer IW (1952) A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem 195:133–140
Bradshaw H, Ceulemans R, Davis J, Stettler R (2000) Emerging model systems in plant biology: poplar (Populus) as a model forest tree. J Plant Growth Regul 19:306–313
Chauhan H, Khurana N, Nijhavan A, Khurana JP, Khurana P (2012) The wheat chloroplastic small heat shock protein (sHSP26) is involved in seed maturation and germination and imparts tolerance to heat stress. Plant Cell Environ 35:1912–1931
Chen H, Lai Z, Shi J, Xiao Y, Chen Z, Xu X (2010) Roles of Arabidopsis WRKY18, WRKY40 and WRKY60 transcription factors in plant responses to abscisic acid and abiotic stress. BMC Plant Biol 10:281
Chung HS, Koo AJ, Gao X, Jayanty S, Thines B, Jones AD, Howe GA (2008) Regulation and function of Arabidopsis JASMONATE ZIM-domain genes in response to wounding and herbivory. Plant Physiol 146:952–964
Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16:735–743
Dang FF, Wang YN, Yu L, Eulgem T, Lai Y, Liu ZQ, Wang X, Qiu AL, Zhang TX, Lin J (2013) CaWRKY40, a WRKY protein of pepper, plays an important role in the regulation of tolerance to heat stress and resistance to Ralstonia solanacearum infection. Plant Cell Environ 36:757–774
De Jong W, Leunissen J, Voorter C (1993) Evolution of the alpha-crystallin/small heat-shock protein family. Mol Biol Evol 10:103–126
del Carmen Martinez-Ballesta M, Carvajal M (2014) New challenges in plant aquaporin biotechnology. Plant Sci 217:71–77
Dubois M, Gilles KA, Hamilton JK, Rebers P, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356
Fujita Y, Fujita M, Shinozaki K, Yamaguchi-Shinozaki K (2011) ABA-mediated transcriptional regulation in response to osmotic stress in plants. J Plant Res 124:509–525
Golldack D, Lüking I, Yang O (2011) Plant tolerance to drought and salinity: stress regulating transcription factors and their functional significance in the cellular transcriptional network. Plant Cell Rep 30:1383–1391
Hu W, Hu G, Han B (2009) Genome-wide survey and expression profiling of heat shock proteins and heat shock factors revealed overlapped and stress specific response under abiotic stresses in rice. Plant Sci 176:583–590
Hubberten H-M, Watanabe M, Bielecka M, Heyneke E, Aarabi F, Hoefgen R (2015) More than a substrate: the O-acetylserine responsive transcriptome. In: Molecular physiology and ecophysiology of sulfur. Springer, Berlin, pp 133–143
Jiang C, Xu J, Zhang H, Zhang X, Shi J, Li M, Ming F (2009) A cytosolic class I small heat shock protein, RcHSP17. 8, of Rosa chinensis confers resistance to a variety of stresses to Escherichia coli, yeast and Arabidopsis thaliana. Plant Cell Environ 32:1046–1059
Kar M, Mishra D (1976) Catalase, peroxidase, and polyphenol oxidase activities during rice leaf senescence. Plant Physiol 57:315–319
Kim DH, Xu Z-Y, Hwang I (2013) AtHSP17.8 overexpression in transgenic lettuce gives rise to dehydration and salt stress resistance phenotypes through modulation of ABA-mediated signaling. Plant Cell Rep 32:1953–1963
Liu B, Wang L, Zhang J, Li J, Zheng H, Chen J, Lu M (2014) WUSCHEL-related Homeobox genes in Populus tomentosa: diversified expression patterns and a functional similarity in adventitious root formation. BMC Genom 15:296
Ma C, Haslbeck M, Babujee L, Jahn O, Reumann S (2006) Identification and characterization of a stress-inducible and a constitutive small heat-shock protein targeted to the matrix of plant peroxisomes. Plant Physiol 141:47–60
Murakami T, Matsuba S, Funatsuki H, Kawaguchi K, Saruyama H, Tanida M, Sato Y (2004) Over-expression of a small heat shock protein, sHSP17.7, confers both heat tolerance and UV-B resistance to rice plants. Mol Breed 13:165–175
Pirkkala L, Nykänen P, Sistonen L (2001) Roles of the heat shock transcription factors in regulation of the heat shock response and beyond. FASEB J 15:1118–1131
Qiu Z, Guo J, Zhu A, Zhang L, Zhang M (2014) Exogenous jasmonic acid can enhance tolerance of wheat seedlings to salt stress. Ecotox Environ Safe 104:202–208
Ruibal C, Castro A, Carballo V, Szabados L, Vidal S (2013) Recovery from heat, salt and osmotic stress in Physcomitrella patens requires a functional small heat shock protein PpHsp16.4. BMC Plant Biol 13:174
Sabehat A, Lurie S, Weiss D (1998) Expression of small heat-shock proteins at low temperatures a possible role in protecting against chilling injuries. Plant Physiol 117:651–658
Smart RE, Bingham GE (1974) Rapid estimates of relative water content. Plant Physiol 53:258–260
Song H, Fan P, Li Y (2009) Overexpression of organellar and cytosolic AtHSP90 in Arabidopsis thaliana impairs plant tolerance to oxidative stress. Plant Mol Biol Rep 27:342–349
Sun W, Bernard C, Van De Cotte B, Van Montagu M, Verbruggen N (2001) At-HSP17.6A, encoding a small heat shock protein in Arabidopsis, can enhance osmotolerance upon overexpression. Plant J 27:407–415
Sun W, Van Montagu M, Verbruggen N (2002) Small heat shock proteins and stress tolerance in plants. BBA Gene Struct Expr 1577:1–9
Sun L, Liu Y, Kong X, Zhang D, Pan J, Zhou Y, Wang L, Li D, Yang X (2012) ZmHSP16. 9, a cytosolic class I small heat shock protein in maize (Zea mays), confers heat tolerance in transgenic tobacco. Plant Cell Rep 31:1473–1484
Topp SD (2008) Regulation of defense responses mediated by Bon1 and Bap2 in Arabidopsis thaliana. Cornell University, Ithaca
Wang F, Dong Q, Jiang H, Zhu S, Chen B, Xiang Y (2012) Genome-wide analysis of the heat shock transcription factors in Populus trichocarpa and Medicago truncatula. Mol Biol Rep 39:1877–1886
Wang A, Yu X, Mao Y, Liu Y, Liu G, Liu Y, Niu X (2015) Overexpression of a small heat-shock-protein gene enhances tolerance to abiotic stresses in rice. Plant Breed 134:384–393
Yan Y, Stolz S, Chételat A, Reymond P, Pagni M, Dubugnon L, Farmer EE (2007) A downstream mediator in the growth repression limb of the jasmonate pathway. Plant Cell 19:2470–2483
Yang H, Yang S, Li Y, Hua J (2007) The Arabidopsis BAP1 and BAP2 genes are general inhibitors of programmed cell death. Plant Physiol 145:135–146
Zhang X, Liu S, Takano T (2008) Two cysteine proteinase inhibitors from Arabidopsis thaliana, AtCYSa and AtCYSb, increasing the salt, drought, oxidation and cold tolerance. Plant Mol Biol 68:131–143
Zhang J, Li J, Liu B, Zhang L, Chen J, Lu M (2013) Genome-wide analysis of the Populus Hsp90 gene family reveals differential expression patterns, localization, and heat stress responses. BMC Genom 14(1):532
Zhang L, Zhang Q, Gao Y, Pan H, Shi S, Wang Y (2014) Overexpression of heat shock protein gene PfHSP21.4 in Arabidopsis thaliana enhances heat tolerance. Acta Physiol Plant 36:1555–1564
Zhang J, Liu B, Li J, Zhang L, Wang Y, Zheng H, Lu M, Chen J (2015) Hsf and Hsp gene families in Populus: genome-wide identification, organization and correlated expression during development and in stress responses. BMC Genom 16(1):1
Zhang J, Chen H, Wang H, Li B, Yi Y, Kong F, Liu J, Zhang H (2016) Constitutive expression of a tomato small heat shock protein gene LeHSP21 improves tolerance to high-temperature stress by enhancing antioxidation capacity in tobacco. Plant Mol Biol Rep 34:399–409
Zhu J-K (2002) Salt and drought stress signal transduction in plants. Annu Rev Plant Biol 53:247
Zhu D, Li R, Liu X, Sun M, Wu J, Zhang N, Zhu Y (2014) The positive regulatory roles of the TIFY10 proteins in plant responses to alkaline stress. PLoS One 9:e111984
Acknowledgments
This work was supported by the China Postdoctoral Science Foundation [2014 M550104] to J.Z. and the National Key Basic Research Program of China [2012CB114500] and a Collaborative Innovation Plan of Jiangsu Higher Education to M.L.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Communicated by K Chong.
J. Li and J. Zhang contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
299_2016_1973_MOESM1_ESM.tif
Fig. S1 The cis- acting elements in promoter of PtHSP17.8 gene were searched in PlantCARE database. The promoter region (1.5 kb upstream to 0.5 kb downstream of the transcription starting site) of PtHSP17.8 was analyzed in PlantCARE database (a). The description and statistics of cis-acting elements were shown in (b). (TIFF 1107 kb)
Rights and permissions
About this article
Cite this article
Li, J., Zhang, J., Jia, H. et al. The Populus trichocarpa PtHSP17.8 involved in heat and salt stress tolerances. Plant Cell Rep 35, 1587–1599 (2016). https://doi.org/10.1007/s00299-016-1973-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-016-1973-3