Abstract
Purpose
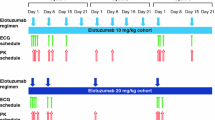
The potential effect of onartuzumab, when administered with or without bevacizumab in combination with weekly paclitaxel, on the corrected QT interval (QTc) and other electrocardiogram (ECG) parameters, was investigated in a randomized, phase 2 study OAM4861g of first- or second-line therapy in patients with locally recurrent or metastatic triple-negative breast cancer.
Methods
Triplicate 12-lead ECGs were recorded at screening, pre- and post-dose on day 1 of cycles 1, 2, and 4, and at the study drug discontinuation visit (SDDV). Onartuzumab serum samples were collected pre- and post-dose on day 1 of cycles 1–4 and at the SDDV. Fridericia’s correction was applied to QT recordings (QTcF), and change from baseline (ΔQTcF) was calculated. Post-baseline measurements were reported as baseline-adjusted control arm (placebo plus bevacizumab plus paclitaxel)-corrected values (ΔΔQTcF). Categorical ECG findings were noted. Linear mixed effects modeling evaluated a potential concentration–ΔQTcF relationship.
Results
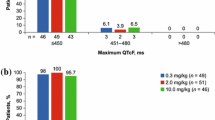
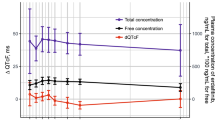
Out of 185 enrolled patients, 165 patients had ECG-evaluable data for analyses. Similar ΔQTcF and ΔΔQTcF values were observed across all treatment arms, with mean increase <10 and <7 ms, respectively, across all time points. Similar changes in heart rate, PR interval, and QRS duration were noted across all treatment arms. Incidences of abnormal ECG findings of clinical interest were comparable in the onartuzumab-containing arms and the control arm. No concentration–ΔQTcF relationship was evident at onartuzumab serum concentrations up to 1,200 μg/ml.
Conclusions
These data suggest that onartuzumab, at the dose and exposures studied in this clinical trial, does not meaningfully affect the QTcF interval.





Similar content being viewed by others
References
Zagouri F, Bago-Horvath Z, Rössler F et al (2013) High MET expression is an adverse prognostic factor in patients with triple-negative breast cancer. Br J Cancer 108:1100–1105
Ho-Yen CM, Green AR, Rakha EA, Brentnall AR, Ellis IO, Kermorgant S, Jones JL (2014) C-Met in invasive breast cancer: is there a relationship with the basal-like subtype? Cancer 120:163–171
Kim JY, Choi JS, Seo J et al (2014) MET is a potential target for use in combination therapy with EGFR inhibition in triple-negative/basal-like breast cancer. Int J Cancer 134:2424–2436
Salgia R, Patel P, Bothos J et al (2014) Phase I dose-escalation study of onartuzumab as a single agent and in combination with bevacizumab in patients with advanced solid malignancies. Clin Cancer Res 20:1666–1675
Spigel DR, Ervin TJ, Ramlau RA et al (2013) Randomized phase II trial of onartuzumab in combination with erlotinib in patients with advanced non-small-cell lung cancer. J Clin Oncol 31:4105–4114
Dieras V, Yardley DA, Romieu G et al (2013) A randomized, phase II, multicenter, double-blind, placebo-controlled trial evaluating onartuzumab with or without bevacizumab in combination with weekly paclitaxel in locally recurrent or metastatic triple-negative breast cancer (TNBC). Cancer Res 73(24 suppl):P2-16-01. doi:10.1158/0008-5472.SABCS13-P2-16-01
European Medicines Agency (2005) The clinical evaluation of QT/QTc interval prolongation and proarrhythmic potential for non-antiarrhythmic drugs E14 (CHMP/ICH/2/04). http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500002879.pdf. Accessed 4 June 2014
International Conference on Harmonization/US Food and Drug Administration Guidance for Industry (2005) E14 clinical evaluation of QT/QTc interval prolongation and proarrhythmic potential for non-antiarrhythmic drugs. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm073153.pdf. Accessed 4 June 2014
Rodriguez I, Erdman A, Padhi D et al (2010) Electrocardiographic assessment for therapeutic proteins—scientific discussion. Am Heart J 160:627–634
Liu Q, Madabushi R, Garnett C, Booth B (2008) Experience in QT evaluation of oncology drug products since ICH E14 guidance. J Clin Oncol 26(15 suppl):125 (Abstract 2554)
Fridericia LS (2003) The duration of systole in an electrocardiogram in normal humans and in patients with heart disease. Ann Noninvasive Electrocardiol 8:343–351
Garnett CE, Beasley N, Bhattaram VA et al (2008) Concentration-QT relationships play a key role in the evaluation of proarrhythmic risk during regulatory review. J Clin Pharmacol 48:13–18
Xin Y, Jin D, Eppler S et al (2013) Population pharmacokinetic analysis from phase I and phase II studies of the humanized monovalent antibody, onartuzumab (MetMAb), in patients with advanced solid tumors. J Clin Pharmacol 53:1103–1111
Bednar MM, Harrigan EP, Anziano RJ, Camm AJ, Ruskin JN (2001) The QT interval. Prog Cardiovasc Dis 43(5 suppl 1):1–45
European Medicines Agency (1997) Anonymous Committee for Proprietary Medicinal Products (CPMP) points to consider: the assessment of the potential for QT interval prolongation by non-cardiovascular medicinal products. http://www.fda.gov/ohrms/dockets/ac/03/briefing/pubs/cpmp.pdf. Accessed 4 June 2014
Rock EP, Finkle J, Fingert HJ et al (2009) Assessing proarrhythmic potential of drugs when optimal studies are infeasible. Am Heart J 157:827–836
Yavas O, Yazici M, Eren O, Oyan B (2007) The acute effect of trastuzumab infusion on ECG parameters in metastatic breast cancer patients. Swiss Med Wkly 137:556–558
Garg A, Li J, Clark E et al (2013) Exposure–response analysis of pertuzumab in HER2-positive metastatic breast cancer: absence of effect on QTc prolongation and other ECG parameters. Cancer Chemother Pharmacol 72:1133–1141
Deeken JF, Shimkus B, Liem A et al (2013) Evaluation of the relationship between cetuximab therapy and corrected QT interval changes in patients with advanced malignancies from solid tumors. Cancer Chemother Pharmacol 71:1473–1483
Taxol® (Paclitaxel) US package insert. http://packageinserts.bms.com/pi/pi_taxol.pdf. Accessed 25 June 2014
Avastin® (Bevacizumab) US package insert. http://www.gene.com/download/pdf/avastin_prescribing.pdf. Accessed 25 June 2014
Acknowledgments
The authors would like to acknowledge the contribution of Melinda Teng in developing an analysis plan and reviewing the ECG analyses. Support for third-party writing assistance for this manuscript was provided by Genentech, Inc.
Conflict of interest
The study was funded by F. Hoffmann-La Roche Ltd., Basel, Switzerland, and Genentech, Inc., a member of the Roche Group, South San Francisco, CA, USA. MTB was a paid consultant of Genentech, Inc. for this project. MC is an employee of F. Hoffmann-La Roche Ltd. SM, WA, SP, and MS are Genentech employees and own Roche stock. IR and JC are Pharsight employees.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Borin, M.T., Chen, M., Mocci, S. et al. Onartuzumab with or without bevacizumab in combination with weekly paclitaxel does not prolong QTc or adversely affect other ECG parameters in patients with locally recurrent or metastatic triple-negative breast cancer. Cancer Chemother Pharmacol 75, 401–410 (2015). https://doi.org/10.1007/s00280-014-2652-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-014-2652-0