Abstract
Purpose
High-dose methotrexate (HD-MTX) has been used to treat children with central nervous system tumors. Accumulation of MTX within pleural, peritoneal, or cardiac effusions has led to delayed excretion and increased risk of systemic toxicity. This retrospective study analyzed the association of intracranial post-resection fluid collections with MTX plasma disposition in infants and young children with brain tumors.
Methods
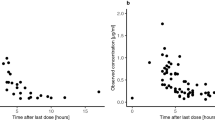
Brain MRI findings were analyzed for postoperative intracranial fluid collections in 75 pediatric patients treated with HD-MTX and for whom serial MTX plasma concentrations (MTX) were collected. Delayed plasma excretion was defined as (MTX) ≥1 μM at 42 hours (h). Leucovorin was administered at 42 h and then every 6 h until (MTX) <0.1 μM. Population and individual MTX pharmacokinetic parameters were estimated by nonlinear mixed-effects modeling.
Results
Fifty-eight patients had intracranial fluid collections present. Population average (inter-individual variation) MTX clearance was 96.0 ml/min/m2 (41.1 CV %) and increased with age. Of the patients with intracranial fluid collections, 24 had delayed excretion; only 2 of the 17 without fluid collections (P < 0.04) had delayed excretion. Eleven patients had grade 3 or 4 toxicities attributed to HD-MTX. No significant difference was observed in intracranial fluid collection, total leucovorin dosing, or hydration fluids between those with and without toxicity.
Conclusions
Although an intracranial fluid collection is associated with delayed MTX excretion, HD-MTX can be safely administered with monitoring of infants and young children with intracranial fluid collections. Infants younger than 1 year may need additional monitoring to avoid toxicity.



Similar content being viewed by others
References
Duffner PK, Horowitz ME, Krischer JP, Friedman HS, Burger PC, Cohen ME et al (1993) Postoperative chemotherapy and delayed radiation in children less than three years of age with malignant brain tumors. N Engl J Med 328:1725–1731
Geyer JR, Zeltzer PM, Boyett JM, Rorke LB, Stanley P, Albright AL et al (1994) Survival of infants with primitive neuroectodermal tumors or malignant ependymomas of the CNS treated with eight drugs in 1 day: a report from the children’s cancer group. J Clin Oncol 12:1607–1615
Geyer JR, Sposto R, Jennings M, Boyett JM, Axtell RA, Breiger D et al (2005) Multiagent chemotherapy and deferred radiotherapy in infants with malignant brain tumors: a report from the children’s cancer group. J Clin Oncol 23:7621–7631
Gajjar A, Mulhern RK, Heideman RL, Sanford RA, Douglass EC, Kovnar EH et al (1994) Medulloblastoma in very young children: outcome of definitive craniospinal irradiation following incomplete response to chemotherapy. J Clin Oncol 12:1212–1216
Walter AW, Mulhern RK, Gajjar A, Heideman RL, Reardon D, Sanford RA et al (1999) Survival and neurodevelopmental outcome of young children with medulloblastoma at St Jude children’s research hospital. J Clin Oncol 17:3720–3728
Rutkowski S, Bode U, Deinlein F, Ottensmeier H, Warmuth-Metz M, Sorensen N et al (2005) Treatment of early childhood medulloblastoma by postoperative chemotherapy alone. N Engl J Med 352:978–986
Chi SN, Gardner SL, Levy AS, Knopp EA, Miller DC, Wisoff JH et al (2004) Feasibility and response to induction chemotherapy intensified with high-dose methotrexate for young children with newly diagnosed high-risk disseminated medulloblastoma. J Clin Oncol 22:4881–4887
Sands SA, Oberg JA, Gardner SL, Whiteley JA, Glade-Bender JL, Finlay JL (2010) Neuropsychological functioning of children treated with intensive chemotherapy followed by myelopablative consolidation chemotherapy and autologous hematopoietic cell rescue for newly diagnosed CNS tumors: an analysis of the Head Start II survivors. Pediatr Blood Cancer 54:429–436
Allen JC, Walker R, Rosen G (1988) Preradiation high-dose intravenous methotrexate with leucovorin rescue for untreated primary childhood brain tumors. J Clin Oncol 6:649–653
Thompson PA, Murry DJ, Rosner GL, Lunagomez S, Blaney SM, Berg SL et al (2007) Methotrexate pharmacokinetics in infants with acute lymphoblastic leukemia. Cancer Chemother Pharmacol 59:847–853
Donelli MG, Zucchetti M, Robatto A, Perlangeli V, D’Incalci M, Masera G et al (1995) Pharmacokinetics of HD-MTX in infants, children, and adolescents with non-B acute lymphoblastic leukemia. Med Pediatr Oncol 24:154–159
Borsi JD, Moe PJ (1987) A comparative study of pharmacokinetics of methotrexate in a dose range of 0.5 g to 33.6 g/m2 in children with acute lymphoblastic leukemia. Cancer 60:5–13
Evans WE, Abromowitch M, Crom WR, Relling MV, Bowman WP, Pui CH et al (1987) Clinical pharmacodynamic studies of high-dose methotrexate in acute lymphocytic leukemia. NCI Monogr 5:81–85
Garre ML, Relling MV, Kalwinsky D, Dodge R, Crom WR, Abromowitch et al (1987) Pharmacokinetics and toxicity of methotrexate in children with down syndrome and acute lymphocytic leukemia. J Pediatr 111:606–614
Am Wall, Gajjar A, Link A, Mahmoud H, Pui CH, Relling MV (2000) Individualized dosing in children with relapsed acute lymphoblastic leukemia. Leukemia 14:221–225
Trevino LR, Shimasaki N, Yang W, Panetta JC, Cheng C, Pei D et al (2009) Germline genetic variation in an organic anion transporter polypeptide associated with methotrexate pharmacokinetic and clinical effects. J Clin Oncol 10:4972–4978
Evans WE, Pratt CB (1978) Effect of pleural effusion on high-dose methotrexate kinetics. Clin Pharmacol Ther 23:68–72
Pauley JL, Panetta JC, Schmidt J, Kornegay N, Relling MV, Pui CH (2004) Late-onset delayed excretion of methotrexate. Cancer Chemother Pharmacol 54:146–152
Li J, Gwilt P (2002) The effect of malignant effusions on methotrexate disposition. Cancer Chemother Pharmacol 50:373–382
Wan SH, Huffman DH, Azarnoff DL, Stephens R, Hoogstraten B (1974) Effect of route of administration and effusions on methotrexate pharmacokinetics. Cancer Res 34:3487–3491
Monolix 3.1: User Guide 2010. http://software.monolix.org
Evans WE, Relling MV, Rodman JH, Crom WR, Boyett JM, Pui CH (1998) Conventional compared with individualized chemotherapy for childhood acute lymphoblastic leukemia. N Engl J Med 338:499–505
Dombrowsky E, Jayaraman B, Narayan M, Barrett JS (2011) Evaluating performance of a decision support system to improve methotrexate pharmacotherapy in children and young adults with cancer. Ther Drug Monit 33:99–107
Aumente D, Buelga DS, Lukas JC, Gomez P, Torres A, Garcia MJ (2006) Population pharmacokinetics of high-dose methotrexate in children with acute lymphoblastic leukaemia. Clin Pharmacokinet 45:1227–1238
Faltaos DW, Hulot JS, Urien S, Morel V, Kaloshi G, Fernandez C, Xuan KH, Leblond V, Lechat P (2006) Population pharmacokinetic study of methotrexate in patients with lymphoid malignancy. Cancer Chemother Pharmacol 58:626–633
Chan H, Evans WE, Pratt CB (1977) Recovery from toxicity associated with high-dose methotrexate: prognostic factors. Cancer Treat Rep 61:797–804
Koizumi H, Fukamachi A, Nukui H (1987) Postoperative subdural fluid collections in neurosurgery. Surg Neurol 27:147–153
Cabanes J, Vazquez R, Rivas A (1978) Hydrocephalus after posterior fossa operations. Surg Neurol 9:42–46
Eguchi S, Aihara Y, Hori T, Okada Y (2011) Postoperative extra-axial cerebrospinal fluid collection –its pathophysiology and clinical management. Pediatr Neurosurg 47:125–132
Gnanalingham KK, Lafuente J, Thompson D, Harkness W, Hayward R (2002) Surgical procedures for posterior fossa tumors in children: does carniotomy lead to fewer complications than craniectomy? J Neurosurg 97:821–826
Ernestus RI, Ketter G, Klug N (1995) Dura-plasty in intracranial operations. Zentralbl Neurochir 56:106–110
Frei E 3rd, Jaffe N, Tattersall MH, Pitman S, Parker L (1975) New approaches to cancer chemotherapy with methotrexate. N Engl J Med 292:846–851
Gandara DR, Edelman MJ, Crowley JJ, Lau DH, Livingston RB (1997) Phase II trial of edatrexate plus carboplatin in metastatic non-small-cell lung cancer: a Southwest oncology group study. Cancer Chemother Pharmacol 41:75–78
Mahadevan A, Kanegaonkar R, Hoskin PJ (1997) Third space sequestration increases toxicity of fludarabine: a case report. Acta Oncol 36:441
Medline Plus: A service of the U.S. National Library of Medicine NIH National Institutes of Health: CSF total protein. http://www.nlm.nih.gov/medlineplus/ency/article/003628.htm
National Cancer Institute Clinical Trials (9/8/2012 update) Phase III study of induction therapy comprising vincristine, high-dose methotrexate, leucovorin calcium, etoposide, cisplatin, and cyclophosphamide followed by 3-dimensional conformal radiotherapy and high-dose consolidation therapy comprising carboplatin, thiotepa, and autologous peripheral blood stem cell rescue in pediatric patients with atypical teratoid/rhabdoid tumor of the central nervous system (ACNS0333). http://cancer.gov/clinicaltrials/search/view?cdrid=592812&version=healthprofessional#StudyIdInfo_CDR0000592812
National Cancer Institute Clinical Trials (9/8/2012 update) Phase III study of induction therapy comprising vincristine, etoposide, cyclophosphamide, and cisplatin with or without high-dose methotrexate and leucovorin calcium followed by consolidation chemotherapy comprising carboplatin and thiotepa and peripheral blood stem cell rescue in pediatric patients with newly diagnosed supratentorial primitive neuroectodermal tumors or high risk medulloblastoma. http://cancer.gov/clinicaltrials/search/view?cdrid=483683&version=HealthProfessional&protocolsearchid=10902453
Bailey CC, Gnekow A, Wellek S, Jones M, Round C, Brown J et al (1991) Prospective randomised trial of chemotherapy given before radiotherapy in childhood medulloblastoma. international society of paediatric oncology (SIOP) and the (German) society of paediatric oncology (GIOP): SIOP II. Med Pediatr Oncol 25:166–178
von Bueren AO, von Hoff K, Pietsch T, Gerber NU, Warmuth-Metz M, Deinlein F et al (2011) Treatment of young children with localized medulloblastoma by chemotherapy alone: results of the prospective, multicenter trial HIT 2000 confirming the prognostic impact of histology. Neuro-Onc 13:669–679
Fangusaro J, Finlay J, Sposto R, Ji L, Saly M, Zacharoulis S et al (2008) Intensive chemotherapy followed by consolidative myeloablative chemotherapy with autologous hematopoietic cell rescue (AuHCR) in young children with newly diagnosed supratentorial primitive neuroectodermal tumors (sPNETs): report of the head start I and II experience. Pediatr Blood Cancer 50:312–318
Gardner SL, Asgharzadeh S, Green A, Horn B, McCowage G, Finlay J (2008) Intensive induction chemotherapy followed by high dose chemotherapy with autologous heamtopoietic progenitor cell rescue in young children newly diagnosed with central nervous system atypical teratoid rhabdoid tumors. Pediatr Blood Cancer 51:235–240
Arant BS Jr (1978) Developmental patterns of renal function maturation compared in the human neonate. J Pediatr 92:705–712
Alcorn J, McNamara PJ (2002) Ontogeny of hepatic and renal systemic clearance pathways in infants. Clin Pharmacokinet 41:1077–1094
Acknowledgments
This work was supported in part by the National Cancer Institute Grants CA21765, CA098543, CA096832, CA154619, and CA081457; Musicians Against Childhood Cancer; the Noyes Brain Tumor Foundation; and the American Lebanese Syrian Associated Charities.
Conflict of interest
The authors have no conflict of interests to disclose and maintain control of all primary data. To that end, the journal may review data as requested.
Ethical Standards
The study was approved by the St. Jude Institutional Review Board and therefore performed with the ethical standards laid down in the 1964 Declaration of Helsinki and it subsequent amendments. All involved patients’ parents gave their informed consents prior to the child’s inclusion in the study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wright, K.D., Panetta, J.C., Onar-Thomas, A. et al. Delayed methotrexate excretion in infants and young children with primary central nervous system tumors and postoperative fluid collections. Cancer Chemother Pharmacol 75, 27–35 (2015). https://doi.org/10.1007/s00280-014-2614-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-014-2614-6