Abstract
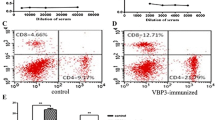
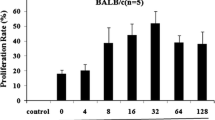
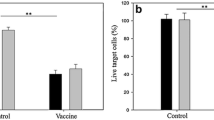
Vascular endothelial growth factor (VEGF) plays an important role in the progression of various cancers. The VEGF-specific antibody bevacizumab combined with chemotherapy was shown to significantly improve progression-free survival in certain cancers. However, repeated administration is necessary for effective suppression of VEGF, thereby making the therapy expensive and cumbersome. Thus, it is urgent to develop alternative reagents such as VEGF vaccines. Here we report that DTT-VEGF, a VEGF-based antigen consisting of the receptor-binding domain of VEGF and diphtheria toxin T domain (DTT), not only stimulated neutralizing antibody response, but also induced type 1 immune response as well as anti-tumor cytotoxic T lymphocytes in mice when administered with aluminum hydroxide adjuvant. The antibodies triggered by DTT-VEGF immunization inhibited the binding of VEGF to VEGF receptor and downregulated the serum VEGF levels in tumor-bearing mice. VEGF-specific IgG2a and IgG2b antibodies as well as type 1 cytokines were stimulated by DTT-VEGF vaccination. The splenocytes from DTT-VEGF-immunized mice showed cytotoxic activity against B16-F10 cells expressing VEGF. Extensive necrosis with severe hemorrhage and enhanced CD8+ T cell infiltration were observed in tumors from DTT-VEGF-immunized mice. The percentages of CD31+ vascular areas in the tumor sections from DTT-VEGF-immunized mice were significantly lower than those of control mice. DTT-VEGF significantly inhibited tumor growth in preventive and therapeutic vaccination settings in mouse models. Our data suggest that DTT is an effective antigen carrier to break immune self-tolerance and our vaccine design has potential to be used for human cancer therapy.






Similar content being viewed by others
Abbreviations
- Alum:
-
Aluminum hydroxide
- CFSE:
-
Carboxyfluorescein succinimidyl ester
- CTLs:
-
Cytotoxic T lymphocytes
- DCs:
-
Dendritic cells
- DTT:
-
Diphtheria toxin T domain
- GST:
-
Glutathione S-transferase
- H&E:
-
Hematoxylin and eosin
- IFA:
-
Incomplete Freund’s adjuvant
- LDH:
-
Lactate dehydrogenase
- mAbs:
-
Monoclonal antibodies
- SEM:
-
Standard error of mean
- VEGF:
-
Vascular endothelial growth factor
- VEGFR:
-
Vascular endothelial growth factor receptor
References
Ferrara N, Kerbel RS (2005) Angiogenesis as a therapeutic target. Nature 438:967–974. doi:10.1038/Nature04483
Mimura K, Kono K, Takahashi A, Kawaguchi Y, Fujii H (2007) Vascular endothelial growth factor inhibits the function of human mature dendritic cells mediated by VEGF receptor-2. Cancer Immunol Immunother 56:761–770. doi:10.1007/s00262-006-0234-7
Ohm JE, Gabrilovich DI, Sempowski GD, Kisseleva E, Parman KS, Nadaf S, Carbone DP (2003) VEGF inhibits T-cell development and may contribute to tumor-induced immune suppression. Blood 101:4878–4886. doi:10.1182/blood-2002-07-1956
Terme M, Pernot S, Marcheteau E, Sandoval F, Benhamouda N, Colussi O, Dubreuil O, Carpentier AF, Tartour E, Taieb J (2013) VEGFA-VEGFR pathway blockade inhibits tumor-induced regulatory T-cell proliferation in colorectal cancer. Cancer Res 73:539–549. doi:10.1158/0008-5472.CAN-12-2325
Griffioen AW (2008) Anti-angiogenesis: making the tumor vulnerable to the immune system. Cancer Immunol Immunother 57:1553–1558. doi:10.1007/s00262-008-0524-3
Miller K, Wang M, Gralow J, Dickler M, Cobleigh M, Perez EA, Shenkier T, Cella D, Davidson NE (2007) Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med 357:2666–2676. doi:10.1056/NEJMoa072113
Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, Lilenbaum R, Johnson DH (2006) Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med 355:2542–2550. doi:10.1056/NEJMoa061884
Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, Ferrara N, Fyfe G, Rogers B, Ross R, Kabbinavar F (2004) Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 350:2335–2342. doi:10.1056/NEJMoa032691
Ebos JM, Kerbel RS (2011) Antiangiogenic therapy: impact on invasion, disease progression, and metastasis. Nat Rev Clin Oncol 8:210–221. doi:10.1038/nrclinonc.2011.21
Wei YQ, Huang MJ, Yang L, Zhao X, Tian L, Lu Y, Shu JM, Lu CJ, Niu T, Kang B, Mao YQ, Liu F, Wen YJ, Lei S, Luo F, Zhou LQ, Peng F, Jiang Y, Liu JY, Zhou H, Wang QR, He QM, Xiao F, Lou YY, Xie XJ, Li Q, Wu Y, Ding ZY, Hu B, Hu M, Zhang W (2001) Immunogene therapy of tumors with vaccine based on Xenopus homologous vascular endothelial growth factor as a model antigen. Proc Natl Acad Sci USA 98:11545–11550
Kyutoku M, Nakagami H, Koriyama H, Tomioka H, Nakagami F, Shimamura M, Kurinami H, Zhengda P, Jo DH, Kim JH, Takakura N, Morishita R (2013) Development of novel DNA vaccine for VEGF in murine cancer model. Sci Rep 3:3380. doi:10.1038/srep03380
Bequet-Romero M, Ayala M, Acevedo BE, Rodriguez EG, Ocejo OL, Torrens I, Gavilondo JV (2007) Prophylactic naked DNA vaccination with the human vascular endothelial growth factor induces an anti-tumor response in C57Bl/6 mice. Angiogenesis 10:23–34. doi:10.1007/s10456-006-9062-9
Wang B, Kaumaya PTP, Cohn DE (2010) Immunization with synthetic VEGF peptides in ovarian cancer. Gynecol Oncol 119:564–570. doi:10.1016/j.ygyno.2010.07.037
Rad FH, Le Buanec H, Paturance S, Larcier P, Genne P, Ryffel B, Bensussan A, Bizzini B, Gallo RC, Zagury D, Uzan G (2007) VEGF kinoid vaccine, a therapeutic approach against tumor angiogenesis and metastases. Proc Natl Acad Sci USA 104:2837–2842. doi:10.1073/pnas.0611022104
Morera Y, Bequet-Romero M, Ayala M, Lamdan H, Agger EM, Andersen P, Gavilondo JV (2008) Anti-tumoral effect of active immunotherapy in C57BL/6 mice using a recombinant human VEGF protein as antigen and three chemically unrelated adjuvants. Angiogenesis 11:381–393. doi:10.1007/s10456-008-9121-5
Kamstock D, Elmslie R, Thamm D, Dow S (2007) Evaluation of a xenogeneic VEGF vaccine in dogs with soft tissue sarcoma. Cancer Immunol Immunother 56:1299–1309. doi:10.1007/s00262-007-0282-7
Hailemichael Y, Dai Z, Jaffarzad N, Ye Y, Medina MA, Huang XF, Dorta-Estremera SM, Greeley NR, Nitti G, Peng W, Liu C, Lou Y, Wang Z, Ma W, Rabinovich B, Schluns KS, Davis RE, Hwu P, Overwijk WW (2013) Persistent antigen at vaccination sites induces tumor-specific CD8(+) T cell sequestration, dysfunction and deletion. Nat Med 19:465–472. doi:10.1038/nm.3105
Salerno EP, Shea SM, Olson WC, Petroni GR, Smolkin ME, McSkimming C, Chianese-Bullock KA, Slingluff CL Jr (2013) Activation, dysfunction and retention of T cells in vaccine sites after injection of incomplete Freund’s adjuvant, with or without peptide. Cancer Immunol Immunother 62:1149–1159. doi:10.1007/s00262-013-1435-5
Chen Z, Dehm S, Bonham K, Kamencic H, Juurlink B, Zhang X, Gordon JR, Xiang J (2001) DNA array and biological characterization of the impact of the maturation status of mouse dendritic cells on their phenotype and antitumor vaccination efficacy. Cell Immunol 214:60–71
Harper SJ, Bates DO (2008) VEGF-A splicing: the key to anti-angiogenic therapeutics? Nat Rev Cancer 8:880–887. doi:10.1038/nrc2505
Christinger HW, Muller YA, Berleau LT, Keyt BA, Cunningham BC, Ferrara N, de Vos AM (1996) Crystallization of the receptor binding domain of vascular endothelial growth factor. Proteins 26:353–357. doi:10.1002/(SICI)1097-0134(199611)26:3<353:AID-PROT9>3.0.CO;2-E
Keyt BA, Berleau LT, Nguyen HV, Chen H, Heinsohn H, Vandlen R, Ferrara N (1996) The carboxyl-terminal domain (111-165) of vascular endothelial growth factor is critical for its mitogenic potency. J Biol Chem 271:7788–7795
Shinefield HR (2010) Overview of the development and current use of CRM(197) conjugate vaccines for pediatric use. Vaccine 28:4335–4339. doi:10.1016/j.vaccine.2010.04.072
Diethelm-Okita BM, Okita DK, Banaszak L, Conti-Fine BM (2000) Universal epitopes for human CD4+ cells on tetanus and diphtheria toxins. J Infect Dis 181:1001–1009. doi:10.1086/315324
Romaniuk SI, Kolybo DV, Komisarenko SV (2012) Recombinant diphtheria toxin derivatives: perspectives of application. Russ J Bioorg Chem 38:565–577. doi:10.1134/s106816201206012x
Choe S, Bennett MJ, Fujii G, Curmi PM, Kantardjieff KA, Collier RJ, Eisenberg D (1992) The crystal structure of diphtheria toxin. Nature 357:216–222. doi:10.1038/357216a0
Hansen B, Sokolovska A, HogenEsch H, Hem SL (2007) Relationship between the strength of antigen adsorption to an aluminum-containing adjuvant and the immune response. Vaccine 25:6618–6624. doi:10.1016/j.vaccine.2007.06.049
Shibuya M (2011) Vascular endothelial growth factor (VEGF) and its receptor (VEGFR) signaling in angiogenesis: a crucial target for anti- and pro-angiogenic therapies. Genes Cancer 2:1097–1105. doi:10.1177/1947601911423031
Melero I, Gaudernack G, Gerritsen W, Huber C, Parmiani G, Scholl S, Thatcher N, Wagstaff J, Zielinski C, Faulkner I, Mellstedt H (2014) Therapeutic vaccines for cancer: an overview of clinical trials. Nat Rev Clin Oncol 11:509–524. doi:10.1038/nrclinonc.2014.111
McGranahan N, Swanton C (2015) Biological and therapeutic impact of intratumor heterogeneity in cancer evolution. Cancer Cell 27:15–26. doi:10.1016/j.ccell.2014.12.001
Schlom J (2012) Therapeutic cancer vaccines: current status and moving forward. J Natl Cancer Inst 104:599–613. doi:10.1093/jnci/djs033
Yadav M, Jhunjhunwala S, Phung QT, Lupardus P, Tanguay J, Bumbaca S, Franci C, Cheung TK, Fritsche J, Weinschenk T, Modrusan Z, Mellman I, Lill JR, Delamarre L (2014) Predicting immunogenic tumour mutations by combining mass spectrometry and exome sequencing. Nature 515:572–576. doi:10.1038/nature14001
Matejuk A, Leng Q, Chou ST, Mixson AJ (2011) Vaccines targeting the neovasculature of tumors. Vasc Cell 3:7. doi:10.1186/2045-824X-3-7
Chen XY, Zhang W, Wu S, Bi F, Su YJ, Tan XY, Liu JN, Zhang J (2006) Vaccination with viable human umbilical vein endothelial cells prevents metastatic tumors by attack on tumor vasculature with both cellular and humoral immunity. Clin Cancer Res 12:5834–5840. doi:10.1158/1078-0432.CCR-06-1105
Poon RT, Fan ST, Wong J (2001) Clinical implications of circulating angiogenic factors in cancer patients. J Clin Oncol 19:1207–1225
Hogenesch H (2012) Mechanism of immunopotentiation and safety of aluminum adjuvants. Front Immunol 3:406. doi:10.3389/fimmu.2012.00406
Spellberg B, Edwards JE Jr (2001) Type 1/Type 2 immunity in infectious diseases. Clin Infect Dis 32:76–102. doi:10.1086/317537
Grivennikov SI, Greten FR, Karin M (2010) Immunity, inflammation, and cancer. Cell 140:883–899. doi:10.1016/j.cell.2010.01.025
Nevala WK, Vachon CM, Leontovich AA, Scott CG, Thompson MA, Markovic SN (2009) Evidence of systemic Th2-driven chronic inflammation in patients with metastatic melanoma. Clin Cancer Res 15:1931–1939. doi:10.1158/1078-0432.CCR-08-1980
Shimato S, Maier LM, Maier R, Bruce JN, Anderson RC, Anderson DE (2012) Profound tumor-specific Th2 bias in patients with malignant glioma. BMC Cancer 12:561. doi:10.1186/1471-2407-12-561
Wang B, Wang X, Wen Y, Fu J, Wang H, Ma Z, Shi Y (2015) Suppression of established hepatocarcinoma in adjuvant only immunotherapy: alum triggers anti-tumor CD8(+) T cell response. Sci Rep 5:17695. doi:10.1038/srep17695
Tsavaris N, Voutsas IF, Kosmas C, Gritzapis AD, Baxevanis CN (2012) Combined treatment with bevacizumab and standard chemotherapy restores abnormal immune parameters in advanced colorectal cancer patients. Invest New Drugs 30:395–402. doi:10.1007/s10637-010-9533-0
Erdag G, Schaefer JT, Smolkin ME, Deacon DH, Shea SM, Dengel LT, Patterson JW, Slingluff CL Jr (2012) Immunotype and immunohistologic characteristics of tumor-infiltrating immune cells are associated with clinical outcome in metastatic melanoma. Cancer Res 72:1070–1080. doi:10.1158/0008-5472.CAN-11-3218
Noble F, Mellows T, McCormick Matthews LH, Bateman AC, Harris S, Underwood TJ, Byrne JP, Bailey IS, Sharland DM, Kelly JJ, Primrose JN, Sahota SS, Bateman AR, Thomas GJ, Ottensmeier CH (2016) Tumour infiltrating lymphocytes correlate with improved survival in patients with oesophageal adenocarcinoma. Cancer Immunol Immunother 65:651–662. doi:10.1007/s00262-016-1826-5
Huang Y, Yuan J, Righi E, Kamoun WS, Ancukiewicz M, Nezivar J, Santosuosso M, Martin JD, Martin MR, Vianello F, Leblanc P, Munn LL, Huang P, Duda DG, Fukumura D, Jain RK, Poznansky MC (2012) Vascular normalizing doses of antiangiogenic treatment reprogram the immunosuppressive tumor microenvironment and enhance immunotherapy. Proc Natl Acad Sci USA 109:17561–17566. doi:10.1073/pnas.1215397109
Flach TL, Ng G, Hari A, Desrosiers MD, Zhang P, Ward SM, Seamone ME, Vilaysane A, Mucsi AD, Fong Y, Prenner E, Ling CC, Tschopp J, Muruve DA, Amrein MW, Shi Y (2011) Alum interaction with dendritic cell membrane lipids is essential for its adjuvanticity. Nat Med 17:479–487. doi:10.1038/nm.2306
Kyrychenko A, Posokhov YO, Rodnin MV, Ladokhin AS (2009) Kinetic intermediate reveals staggered pH-dependent transitions along the membrane insertion pathway of the diphtheria toxin T-domain. Biochemistry 48:7584–7594. doi:10.1021/bi9009264
Acknowledgements
This work was supported by National S and T major projects of China (Key Innovative Drug Development) (No. 2014ZX09101043), Shanghai Municipal Science and Technology Program (No. 14431904100), Shanghai Industry-Academia-Research Collaboration Program (No. CXY-2013-54), Medicine Science/Engineering Hybrid Project of Shanghai Jiao Tong University (No. YG2013MS09) and Specialized Research Fund for the Doctoral Program of Higher Education of China (SRFDP) (No. 20130073120110).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Xu, A., Zhang, L., Chen, Y. et al. Immunogenicity and efficacy of a rationally designed vaccine against vascular endothelial growth factor in mouse solid tumor models. Cancer Immunol Immunother 66, 181–192 (2017). https://doi.org/10.1007/s00262-016-1928-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-016-1928-0