Abstract
Purpose
This translational study is designed to assess the safety, dosimetry and therapeutic response to a single, low-dose of 177Lu-EB-PSMA-617 in comparison to 177Lu-PSMA-617 in patients with mCRPC.
Methods
Following institutional review board approval and informed consent, nine patients with mCRPC were recruited. Four patients accepted intravenous injection of 0.80–1.1 GBq (21.5–30 mCi) of 177Lu-EB-PSMA-617, then underwent serial whole-body planar and SPECT/CT imaging at 2, 24, 72, 120 and 168 h. The other five patients accepted intravenous injection of 1.30–1.42 GBq (35–38.4 mCi) 177Lu-PSMA-617, then underwent the same imaging procedures at 0.5, 2, 24, 48, and 72 h. All patients were evaluated by 68Ga-PSMA-617 PET/CT before and one month after the treatment. Dosimetry evaluation was compared in both patient groups.
Results
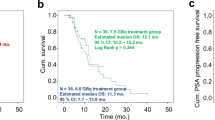
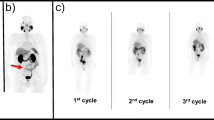
When the bone metastasis tumors with comparable baseline SUVmax in the range of 10.0–15.0 were selected from the two groups for comparison, the accumulated radioactivity of 177Lu-EB-PSMA-617 was about 3.02-fold higher than that of 177Lu-PSMA-617. Imaging dose of 177Lu-EB-PSMA-617 treatment showed significant decrease of 68Ga-PSMA-617 uptake within a month, which was not observed in patients imaged with 177Lu-PSMA-617 (SUV change: −32.43 ± 0.14% vs. 0.21 ± 0.37%; P = 0.002). 177Lu-EB-PSMA-617 also had higher absorbed doses in the red bone marrow and kidneys than 177Lu-PSMA-617 (0.0547 ± 0.0062 vs. 0.0084 ± 0.0057 mSv/MBq for red bone marrow, P < 0.01; 2.39 ± 0.69 vs. 0.39 ± 0.06 mSv/MBq for kidneys, P < 0.01).
Conclusion
This first-in-human study demonstrated that 177Lu-EB-PSMA-617 had higher accumulation in mCRPC and that low imaging dose appears to be effective in treating tumors with high 68Ga-PSMA-617 uptakes. Elevated uptakes of 177Lu-EB-PSMA-617 in kidneys and red bone marrow were well tolerated at the administered low dose. Further investigations with increased dose and frequency of administration are warranted.



Similar content being viewed by others
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30.
Kirby M, Hirst C, Crawford ED. Characterising the castration-resistant prostate cancer population: a systematic review. Int J Clin Pract. 2011;65:1180–92.
Cornford P, Bellmunt J, Bolla M, et al. EAU-ESTRO-SIOG guidelines on PC. Part II: treatment of relapsing, metastatic, and CRPC. Eur Urol. 2017;71:630–42.
Heidenreich A, Bastian PJ, Bellmunt J, et al. EAU guidelines on prostate cancer. Part 1: screening, diagnosis, and local treatment with curative intent—update 2013. Eur Urol. 2014;65:124–37.
Rajasekaran AK, Anilkumar G, Christiansen JJ. Is prostate specific membrane antigen a multifunctional protein? Am J Physiol Cell Physiol. 2005;288:C975–81.
Ghosh A, Heston WD. Tumor target prostate specific membrane antigen (PSMA) and its regulation in prostate cancer. J Cell Biochem. 2004;91:528–39.
Perner S, Hofer MD, Kim R, et al. Prostate-specific membrane antigen expression as a predictor of prostate cancer progression. Hum Pathol. 2007;38:696–701.
Afshar-Oromieh A, Malcher A, Eder M, et al. PET imaging with a [68Ga]gallium-labelled PSMA ligand for the diagnosis of PCa: biodistribution in humans and first evaluation of tumour lesions. Eur J Nucl Med Mol Imaging. 2013;40:486–95.
Wright GL Jr, Haley C, Beckett ML, et al. Expression of prostate-specific membrane antigen in normal, benign and malignant prostate tissues. Urol Oncol. 1995;1:18–28.
Benesová M, Schafer M, Bauder-Wust U, et al. Preclinical evaluation of a tailor made DOTA-conjugated PSMA inhibitor with optimized linker moiety for imaging and endoradiotherapy of prostate cancer. J Nucl Med. 2015;56:914–20.
Yadav MP, Ballal S, Tripathi M, et al. 177Lu-DKFZ-PSMA-617 therapy in metastatic castration resistant prostate cancer: Safety, efficacy, and quality of life assessment. Eur J Nucl Med Mol Imaging. 2016;44:81–91.
Rahbar K, Ahmadzadehfar H, Kratochwil C, et al. German multicenter study investigating 177Lu-PSMA-617 radioligand therapy in advanced prostate cancer patients. J Nucl Med. 2017;58(1):85–90.
Jacobson O, Wang Z, Niu G, Ma Y, Kiesewetter DO, Chen X. A single injection of Evans blue modified 177Lu-PSMA-617 provides a radiotherapeutic cure for prostate-specific membrane antigen (PSMA) tumor xenografts in mice. J Nucl Med. 2018.
Common Terminology Criteria for Adverse Events v3.0 (CTCAE). cancer.gov Website. http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf. Published August 9, 2006. Accessed April 14, 2016.
Lassmann M, Chiesa C, Flux G, Bardies M. EANM Dosimetry Committee guidance document: good practice of clinical dosimetry reporting. Eur J Nucl Med Mol Imaging. 2011;38:192–200.
Roivainen A, Kähkönen E, Luoto P, et al. Plasma pharmacokinetics, whole-body distribution, metabolism, and radiation dosimetry of 68Ga bombesin antagonist BAY 86-7548 in healthy men. J Nucl Med. 2013;54:867–72.
Wang Z, Zhang M, Wang L, et al. Prospective study of 68Ga-NOTA-NFB: radiation dosimetry in healthy volunteers and first application in glioma patients. Theranostics. 2015;5:882–9.
Wessels BW, Bolch WE, Bouchet LG, et al. Bone marrow dosimetry using blood-based models for radiolabeled antibody therapy: a multiinstitutional comparison. J Nucl Med. 2004;45:1725–33.
Kratochwil C, Giesel FL, Stefanova M, et al. PSMA-targeted radionuclide therapy of metastatic castration-resistant prostate cancer with Lu-177 labeled PSMA-617. J Nucl Med. 2016;57:1170–6.
Minner S, Wittmer C, Graefen M, et al. High level PSMA expression is associated with early PSA recurrence in surgically treated prostate cancer. Prostate. 2011;71:281–8.
Rybalov M, Ananias HJ, Hoving HD, et al. PSMA, EpCAM, VEGF and GRPR as imaging targets in locally recurrent prostate cancer after radiotherapy. Int J Mol Sci. 2014;15:6046–61.
Ananias HJ, van den Heuvel MC, Helfrich W, et al. Expression of the gastrin-releasing peptide receptor, the prostate stem cell antigen and the prostate-specific membrane antigen in lymph node and bone metastases of prostate cancer. Prostate. 2009;69:1101–8.
Ahmadzadehfar H, Eppard E, Kurpig S, et al. Therapeutic response and side effects of repeated radioligand therapy with 177Lu-PSMA-DKFZ-617 of castrate-resistant metastatic prostate cancer. Oncotarget. 2016;7:12477–88.
Rahbar K, Schmidt M, Heinzel A, et al. Response and tolerability of a single dose of 177Lu-PSMA-617 in patients with metastatic castration-resistant prostate cancer: a multicenter retrospective analysis. J Nucl Med. 2016;57:1334–8.
Rahbar K, Bode A, Weckesser M, et al. Radioligand therapy with 177Lu-PSMA-617 as a novel therapeutic option in patients with metastatic castration resistant prostate cancer. Clin Nucl Med. 2016;41:522–8.
Emmett L, Willowson K, Violet J, et al. Lutetium 177PSMA radionuclide therapy for men with prostate cancer: a review of the current literature and discussion of practical aspects of therapy. J Med Radiat Sci. 2017;64(1):52–60.
Kulkarni HR, Singh A, Schuchardt C, et al. PSMA-based radioligand therapy for metastatic castration-resistant prostate cancer: the Bad Berka experience since 2013. J Nucl Med. 2016;57(Suppl 3):97S–104S.
Delker A, Fendler WP, Kratochwil C, et al. Dosimetry for Lu-DKFZ-PSMA-617: a new radiopharmaceutical for the treatment of metastatic prostate cancer. Eur J Nucl Med Mol Imaging. 2016;43:42–51.
Jeong SY, Kim HW, Lee SW, Ahn BC, Lee J. Salivary gland function 5 years after radioactive iodine ablation in patients with differentiated thyroid cancer: direct comparison of pre- and postablation scintigraphies and their relation to xerostomia symptoms. Thyroid. 2013;23:609–16.
Funding
This study was supported in part by the Key Project on Inter-Governmental International Scientific and Technological Innovation Cooperation in National Key Projects of Research and Development Plan (2016YFE0115400), the Intramural Research Program (IRP), National Institute of Biomedical Imaging and Bioengineering (NIBIB), and National Institutes of Health (NIH). This study was also partly supported by the Chinese Academy of Medical Science Major Collaborative Innovation Project (2016-I2M-1-011), Welfare Research Funding for Public Health Professionals (201402001), National Nature Science Foundation (81741142) and Beijing Municipal Natural Science Foundation (7161012).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Rights and permissions
About this article
Cite this article
Zang, J., Fan, X., Wang, H. et al. First-in-human study of 177Lu-EB-PSMA-617 in patients with metastatic castration-resistant prostate cancer. Eur J Nucl Med Mol Imaging 46, 148–158 (2019). https://doi.org/10.1007/s00259-018-4096-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-018-4096-y