Abstract
Purpose
We estimated whether amyloid involvement in subcortical regions may predict cognitive impairment, and established an amyloid staging scheme based on degree of subcortical amyloid involvement.
Methods
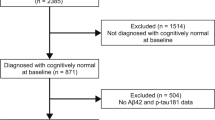
Data from 240 cognitively normal older individuals, 393 participants with mild cognitive impairment, and 126 participants with Alzheimer disease were acquired at Alzheimer’s Disease Neuroimaging Initiative sites. To assess subcortical involvement, we analyzed amyloid deposition in amygdala, putamen, and caudate nucleus. We staged participants into a 3-stage model based on cortical and subcortical amyloid involvement: 382 with no cortical or subcortical involvement as stage 0, 165 with cortical but no subcortical involvement as stage 1, and 203 with both cortical and subcortical involvement as stage 2.
Results
Amyloid accumulation was first observed in cortical regions and spread down to the putamen, caudate nucleus, and amygdala. In longitudinal analysis, changes in MMSE, ADAS-cog 13, FDG PET SUVR, and hippocampal volumes were steepest in stage 2 followed by stage 1 then stage 0 (p value <0.001). Stage 2 showed steeper changes in MMSE score (β [SE] = −0.02 [0.004], p < 0.001), ADAS-cog 13 (0.05 [0.01], p < 0.001), FDG PET SUVR (−0.0008 [0.0003], p = 0.004), and hippocampal volumes (−4.46 [0.65], p < 0.001) compared to stage 1.
Conclusions
We demonstrated a downward spreading pattern of amyloid, suggesting that amyloid accumulates first in neocortex followed by subcortical structures. Furthermore, our new finding suggested that an amyloid staging scheme based on subcortical involvement might reveal how differential regional accumulation of amyloid affects cognitive decline through functional and structural changes of the brain.





Similar content being viewed by others
References
Jack CR Jr, Knopman DS, Jagust WJ, Petersen RC, Weiner MW, Aisen PS, et al. Tracking pathophysiological processes in Alzheimer's disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013;12:207–16.
Jack CR Jr, Knopman DS, Jagust WJ, Shaw LM, Aisen PS, Weiner MW, et al. Hypothetical model of dynamic biomarkers of the Alzheimer's pathological cascade. Lancet Neurol. 2010;9:119–28.
Thal DR, Rub U, Orantes M, Braak H. Phases of a beta-deposition in the human brain and its relevance for the development of AD. Neurology. 2002;58:1791–800.
Braak H, Braak E. Alzheimer's disease: striatal amyloid deposits and neurofibrillary changes. J Neuropathol Exp Neurol. 1990;49:215–24.
Beach TG, Sue LI, Walker DG, Sabbagh MN, Serrano G, Dugger BN, et al. Striatal amyloid plaque density predicts Braak neurofibrillary stage and clinicopathological Alzheimer's disease: implications for amyloid imaging. J Alzheimers Dis. 2012;28:869–76.
Thal DR, Beach TG, Zanette M, Heurling K, Chakrabarty A, Ismail A, et al. [(18)F]flutemetamol amyloid positron emission tomography in preclinical and symptomatic Alzheimer's disease: specific detection of advanced phases of amyloid-beta pathology. Alzheimers Dement. 2015;11:975–85.
Wolf DS, Gearing M, Snowdon DA, Mori H, Markesbery WR, Mirra SS. Progression of regional neuropathology in Alzheimer disease and normal elderly: findings from the Nun study. Alzheimer Dis Assoc Disord. 1999;13:226–31.
Cho H, Choi JY, Hwang MS, Kim YJ, Lee HM, Lee HS, et al. In vivo cortical spreading pattern of tau and amyloid in the Alzheimer disease spectrum. Ann Neurol. 2016;80:247–58.
Seo SW, Ayakta N, Grinberg LT, Villeneuve S, Lehmann M, Reed B, et al. Regional correlations between [11C]PIB PET and post-mortem burden of amyloid-beta pathology in a diverse neuropathological cohort. Neuroimage Clin. 2017;13:130–7.
Stepan-Buksakowska I, Szabo N, Horinek D, Toth E, Hort J, Warner J, et al. Cortical and subcortical atrophy in Alzheimer disease: parallel atrophy of thalamus and hippocampus. Alzheimer Dis Assoc Disord. 2014;28:65–72.
Ma X, Li Z, Jing B, Liu H, Li D, Li H, et al. Identify the atrophy of Alzheimer's disease, mild cognitive impairment and normal aging using morphometric MRI analysis. Front Aging Neurosci. 2016;8:243.
Doan NT, Engvig A, Zaske K, Persson K, Lund MJ, Kaufmann T, et al. Distinguishing early and late brain aging from the Alzheimer's disease spectrum: consistent morphological patterns across independent samples. Neuroimage. 2017;158:282–95.
Chung SJ, Shin JH, Cho KH, Lee Y, Sohn YH, Seong JK, et al. Subcortical shape analysis of progressive mild cognitive impairment in Parkinson's disease. Mov Disord. 2017;32:1447–56.
Koo DL, Shin JH, Lim JS, Seong JK, Joo EY. Changes in subcortical shape and cognitive function in patients with chronic insomnia. Sleep Med. 2017;35:23–6.
Petersen RC, Aisen PS, Beckett LA, Donohue MC, Gamst AC, Harvey DJ, et al. Alzheimer's disease neuroimaging initiative (ADNI): clinical characterization. Neurology. 2010;74:201–9.
Hsu YY, Schuff N, Du AT, Mark K, Zhu X, Hardin D, et al. Comparison of automated and manual MRI volumetry of hippocampus in normal aging and dementia. J Magn Reson Imaging. 2002;16:305–10.
Landau SM, Harvey D, Madison CM, Koeppe RA, Reiman EM, Foster NL, et al. Associations between cognitive, functional, and FDG-PET measures of decline in AD and MCI. Neurobiol Aging. 2011;32:1207–18.
Cho Y, Seong JK, Shin SY, Jeong Y, Kim JH, Qiu AQ, et al. A multi-resolution scheme for distortion-minimizing mapping between human subcortical structures based on geodesic construction on Riemannian manifolds. Neuroimage. 2011;57:1376–92.
Cho Y, Seong JK, Jeong Y, Shin SY. Alzheimer's disease neuroimaging I. Individual subject classification for Alzheimer's disease based on incremental learning using a spatial frequency representation of cortical thickness data. Neuroimage. 2012;59:2217–30.
Landau SM, Breault C, Joshi AD, Pontecorvo M, Mathis CA, Jagust WJ, et al. Amyloid-beta imaging with Pittsburgh compound B and florbetapir: comparing radiotracers and quantification methods. J Nucl Med. 2013;54:70–7.
Grothe MJ, Barthel H, Sepulcre J, Dyrba M, Sabri O, Teipel SJ. In vivo staging of regional amyloid deposition. Neurology. 2017;89:2031–8.
Hanseeuw BJ, Betensky RA, Mormino EC, Schultz AP, Sepulcre J, Becker JA, et al. PET staging of amyloidosis using striatum. Alzheimers Dement. 2018.
Beach TG, Thal DR, Zanette M, Smith A, Buckley C. Detection of striatal amyloid plaques with [18F]flutemetamol: validation with postmortem histopathology. J Alzheimers Dis. 2016;52:863–73.
Shah N, Frey KA, Muller ML, Petrou M, Kotagal V, Koeppe RA, et al. Striatal and cortical beta-Amyloidopathy and cognition in Parkinson's disease. Mov Disord. 2016;31:111–7.
Dickerson BC, Wolk DA. Biomarker-based prediction of progression in MCI: comparison of AD signature and hippocampal volume with spinal fluid amyloid-β and tau. Front Aging Neurosci. 2013;5.
Landau SM, Mintun MA, Joshi AD, Koeppe RA, Petersen RC, Aisen PS, et al. Amyloid deposition, hypometabolism, and longitudinal cognitive decline. Ann Neurol. 2012;72:578–86.
Okello A, Koivunen J, Edison P, Archer HA, Turkheimer FE, Nagren K, et al. Conversion of amyloid positive and negative MCI to AD over 3 years: an 11C-PIB PET study. Neurology. 2009;73:754–60.
Doraiswamy PM, Sperling RA, Johnson K, Reiman EM, Wong TZ, Sabbagh MN, et al. Florbetapir F 18 amyloid PET and 36-month cognitive decline: a prospective multicenter study. Mol Psychiatry. 2014;19:1044–51.
Klunk WE, Price JC, Mathis CA, Tsopelas ND, Lopresti BJ, Ziolko SK, et al. Amyloid deposition begins in the striatum of presenilin-1 mutation carriers from two unrelated pedigrees. J Neurosci. 2007;27:6174–84.
Funding
This research was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIP) (No. NRF-2017R1A2B2005081 and No. 2016R1A2B4014398) and National Institutes of Health (NIH) grant P30AG049638.
Author information
Authors and Affiliations
Consortia
Contributions
S.H.C., J.H.S., J.K.S. and S.W.S. contributed to the conceptualization of the study, analysis and interpretation of data, and drafting. J.H.S. and S.P contributed to analyses of imaging data, prepared the figures, and provided technical support. H.J.K., H.M.J., S.E.K., S.J.K., Y.K. and J.S.L. contributed to interpretation of data. S.L., G.D.R. and D.L.N. contributed to analysis and interpretation of data.
Corresponding authors
Ethics declarations
Conflict of interest
All authors have no conflicts of interest to disclose.
Role of the funder
The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Electronic supplementary material
ESM 1
(DOCX 336 kb)
Rights and permissions
About this article
Cite this article
Cho, S.H., Shin, JH., Jang, H. et al. Amyloid involvement in subcortical regions predicts cognitive decline. Eur J Nucl Med Mol Imaging 45, 2368–2376 (2018). https://doi.org/10.1007/s00259-018-4081-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-018-4081-5