Abstract
Introduction
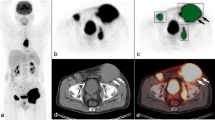
Our aim was to study the prognostic value of two new 18F-FDG PET biomarkers in diffuse large B-cell lymphoma (DLBCL). We examined the total tumor surface (TTS), describing the tumor–host interface, and the tumor volume surface ratio (TVSR), corresponding to the ratio between the total metabolic tumor volume (TMTV) and TTS, describing the tumor fragmentation.
Methods
We retrospectively included 215 patients with DLBCL. Patients underwent initial 18F-FDG PET/CT before R-CHOP (73%) or intensified R-CHOP (R-ACVBP) regimens (27%). The TMTV was measured using a fixed threshold value of 41% of SUVmax. To calculate TTS and TVSR, the surface was measured using an in-house software based on the marching cube algorithm. Spearman’s rank correlation coefficient (ρ) was computed between TMTV, TTS, and TVSR, and ROC analysis was performed. Survival functions at 5 years were studied using a Kaplan-Meier method and uni/multivariate Cox analysis.
Results
TVSR was poorly correlated with TMTV (ρ = 0.5) and TTS (ρ = 0.26), while TTS was highly correlated with TMTV (ρ = 0.94) and was, therefore, excluded from the analysis. TMTV had the highest area under the ROC curve (0.711) and the best sensitivity (0.797), while TVSR had the best specificity (0.745). The optimal cut-off values to predict 5-year OS were 222 cm3 for TMTV and 6.0 cm for TVSR. Patients with high TMTV and TVSR had significantly worse prognosis in Kaplan-Meier and Cox univariate analysis. In a multivariate Cox analysis combining the International Prognostic Index (IPI), the type of chemotherapy, TMTV, and TVSR, all parameters were independent and significant prognostic factors (HR [95%CI]: IPI 1.4 [1.1-1.8], type of chemotherapy 4.5 [2.0-10.5], TMTV 2.8 [1.4-5.5], TVSR 2.1 [1.3-3.4]). A synergistic effect between TMTV and TVSR was observed in a Kaplan-Meier analysis combining the two parameters.
Conclusions
TVSR measured on the initial 18F-FDG PET is an independent prognostic factor in DLBCL and has an additional prognostic value when combined with TMTV, IPI score and chemotherapy.



Similar content being viewed by others
Change history
26 May 2018
A unit error concerning the tumor volume surface ratio (TVSR) is present throughout the article. The unit reported is "cm" but is actually "mm".
References
Tilly H, Gomes da Silva M, Vitolo U, Jack A, Meignan M, Lopez-Guillermo A, et al. Diffuse large B-cell lymphoma (DLBCL): ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26(Suppl 5):v116–25.
Coiffier B, Thieblemont C, Van Den Neste E, Lepeu G, Plantier I, Castaigne S, et al. Long-term outcome of patients in the LNH-98.5 trial, the first randomized study comparing rituximab-CHOP to standard CHOP chemotherapy in DLBCL patients: a study by the Groupe d’Etudes des Lymphomes de l’Adulte. Blood. 2010;116:2040–5.
Habermann TM, Weller EA, Morrison VA, Gascoyne RD, Cassileth PA, Cohn JB, et al. Rituximab-CHOP versus CHOP alone or with maintenance rituximab in older patients with diffuse large B-cell lymphoma. J Clin Oncol. 2006;24:3121–7.
Récher C, Coiffier B, Haioun C, Molina TJ, Fermé C, Casasnovas O, et al. Intensified chemotherapy with ACVBP plus rituximab versus standard CHOP plus rituximab for the treatment of diffuse large B-cell lymphoma (LNH03-2B): an open-label randomised phase 3 trial. Lancet. 2011;378:1858–67.
International Non-Hodgkin’s Lymphoma Prognostic Factors Project. A predictive model for aggressive non-Hodgkin’s lymphoma. N Engl J Med. 1993;329:987–94.
Zhou Z, Sehn LH, Rademaker AW, Gordon LI, Lacasce AS, Crosby-Thompson A, et al. An enhanced international prognostic index (NCCN-IPI) for patients with diffuse large B-cell lymphoma treated in the rituximab era. Blood. 2014;123:837–42.
Meignan M, Sasanelli M, Casasnovas RO, Luminari S, Fioroni F, Coriani C, et al. Metabolic tumour volumes measured at staging in lymphoma: methodological evaluation on phantom experiments and patients. Eur J Nucl Med Mol Imaging. 2014;41:1113–22.
Barrington SF, Kluge R. FDG PET for therapy monitoring in Hodgkin and non-Hodgkin lymphomas. Eur J Nucl Med Mol Imaging. 2017;44:97–110.
Barrington SF, Mikhaeel NG, Kostakoglu L, Meignan M, Hutchings M, Müeller SP, et al. Role of imaging in the staging and response assessment of lymphoma: consensus of the international conference on malignant lymphomas imaging working group. J Clin Oncol. 2014;32:3048–58.
Sasanelli M, Meignan M, Haioun C, Berriolo-Riedinger A, Casasnovas R-O, Biggi A, et al. Pretherapy metabolic tumour volume is an independent predictor of outcome in patients with diffuse large B-cell lymphoma. Eur J Nucl Med Mol Imaging. 2014;41:2017–22.
Song M-K, Chung J-S, Shin H-J, Lee S-M, Lee S-E, Lee H-S, et al. Clinical significance of metabolic tumor volume by PET/CT in stages II and III of diffuse large B cell lymphoma without extranodal site involvement. Ann Hematol. 2012;91:697–703.
Kim J, Hong J, Kim SG, Hwang KH, Kim M, Ahn HK, et al. Prognostic value of metabolic tumor volume estimated by (18) F-FDG positron emission tomography/computed tomography in patients with diffuse large B-cell lymphoma of stage II or III disease. Nucl Med Mol Imaging. 2014;48:187–95.
Xie M, Wu K, Liu Y, Jiang Q, Xie Y. Predictive value of F-18 FDG PET/CT quantization parameters in diffuse large B cell lymphoma: a meta-analysis with 702 participants. Med Oncol. 2015;32:446.
Xie M, Zhai W, Cheng S, Zhang H, Xie Y, He W. Predictive value of F-18 FDG PET/CT quantization parameters for progression-free survival in patients with diffuse large B-cell lymphoma. Hematology. 2016;21:99–105.
Cottereau A-S, Lanic H, Mareschal S, Meignan M, Vera P, Tilly H, et al. Molecular profile and FDG-PET/CT total metabolic tumor volume improve risk classification at diagnosis for patients with Diffuse Large B Cell Lymphoma. Clin Cancer Res. 2016; clincanres.2825.2015.
Rachinel N, Salles G. The host-tumor interface in B-cell non-Hodgkin lymphoma: a new world to investigate. Curr Hematol Malig Rep. 2009;4:196–201.
Lorensen WE, Cline HE. Marching Cubes: A High Resolution 3D Surface Construction Algorithm. Proceedings of the 14th Annual Conference on Computer Graphics and Interactive Techniques [Internet]. New York, NY, USA: ACM; 1987. p. 163–169.
Tilly H, Lepage E, Coiffier B, Blanc M, Herbrecht R, Bosly A, et al. Intensive conventional chemotherapy (ACVBP regimen) compared with standard CHOP for poor-prognosis aggressive non-Hodgkin lymphoma. Blood. 2003;102:4284–9.
Boellaard R, Delgado-Bolton R, Oyen WJG, Giammarile F, Tatsch K, Eschner W, et al. FDG PET/CT: EANM procedure guidelines for tumour imaging: version 2.0. Eur J Nucl Med Mol Imaging. 2015;42:328–54.
Rao L, Wang X, Zong Z, Chen Z, Shi X, Yi C, et al. PET-CT for Evaluation of Spleen and Liver 18F-FDG Diffuse Uptake Without Lymph Node Enlargement in Lymphoma. Medicine (Baltimore). 2016;95.
Cheson BD, Pfistner B, Juweid ME, Gascoyne RD, Specht L, Horning SJ, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25:579–86.
Team RDC. R: a language and environment for statistical computing [internet]. R Foundation for Statistical Computing: Vienna; 2008. Available from: http://www.R-project.org
Chalkidou A, O’Doherty MJ, Marsden PK. False discovery rates in PET and CT studies with texture features: a systematic review. PLoS One. 2015;10:e0124165.
Toledano MN, Desbordes P, Banjar A, Gardin I, Vera P, Ruminy P, et al. Combination of baseline FDG PET/CT total metabolic tumour volume and gene expression profile have a robust predictive value in patients with diffuse large B-cell lymphoma. Eur J Nucl Med Mol Imaging. 2018;
Ilyas H, Mikhaeel NG, Dunn JT, Rahman F, Møller H, Smith D, et al. Defining the optimal method for measuring baseline metabolic tumour volume in diffuse large B cell lymphoma. Eur J Nucl Med Mol Imaging. 2018.
Ben Bouallègue F, Tabaa YA, Kafrouni M, Cartron G, Vauchot F, Mariano-Goulart D. Association between textural and morphological tumor indices on baseline PET-CT and early metabolic response on interim PET-CT in bulky malignant lymphomas. Med Phys. 2017;44:4608–19.
Bukovsky A, Caudle MR, Wimalasena J, Keenan JA, Elder RF. The role of the host-tumor interface and cell hybridization in invasive cancer. Med Hypotheses. 2001;57:729–35.
Yu Y, Decazes P, Gardin I, Vera P, Ruan S. 3D Lymphoma Segmentation in PET/CT Images Based on Fully Connected CRFs. Molecular Imaging, Reconstruction and Analysis of Moving Body Organs, and Stroke Imaging and Treatment. Springer, Cham; 2017.
Grossiord É, Talbot H, Passat N, Meignan M, Najman L. Automated 3D lymphoma lesion segmentation from PET/CT characteristics. 2017 I.E. 14th International Symposium on Biomedical Imaging (ISBI 2017). 2017. p. 174–8.
Cottereau AS, Versari A, Luminari S, Dupuis J, Chartier L, Casasnovas R-O, et al. Prognostic model for high tumor burden follicular lymphoma integrating baseline and end induction PET: a LYSA/FIL study. Blood. 2018.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This study was performed in accordance with the Helsinki Declaration and local laws, and the protocol was approved by the Institutional Review Board of Henri Becquerel Centre.
Inform consent
All patients participating in this study provided written informed consent.
Electronic supplementary material
ESM 1
(DOCX 110 kb)
Rights and permissions
About this article
Cite this article
Decazes, P., Becker, S., Toledano, M. et al. Tumor fragmentation estimated by volume surface ratio of tumors measured on 18F-FDG PET/CT is an independent prognostic factor of diffuse large B-cell lymphoma. Eur J Nucl Med Mol Imaging 45, 1672–1679 (2018). https://doi.org/10.1007/s00259-018-4041-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-018-4041-0