Abstract
Purpose
We aim to report the quality of accuracy studies investigating the utility of [18F]fluorodeoxyglucose (FDG)-PET in supporting the diagnosis of prodromal Alzheimer’s Disease (AD), frontotemporal lobar degeneration (FTLD) and prodromal dementia with Lewy bodies (DLB) in mild cognitive impairment (MCI) subjects, and the corresponding recommendations made by a panel of experts.
Methods
Seven panellist, four from the European Association of Nuclear Medicine, and three from the European Academy of Neurology, produced recommendations taking into consideration the incremental value of FDG-PET, as added on clinical-neuropsychological examination, to ascertain the aetiology of MCI (AD, FTLD or DLB). A literature search using harmonized population, intervention, comparison, and outcome (PICO) strings was performed, and an evidence assessment consistent with the European Federation of Neurological Societies guidance was provided. The consensual recommendation was achieved based on Delphi rounds.
Results
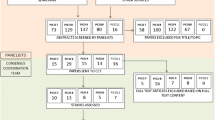
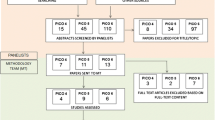
Fifty-four papers reported the comparison of interest. The selected papers allowed the identification of FDG patterns that characterized MCI due to AD, FTLD and DLB. While clinical outcome studies supporting the diagnosis of MCI due to AD showed varying accuracies (ranging from 58 to 100%) and varying areas under the receiver-operator characteristic curves (0.66 to 0.97), no respective data were identified for MCI due to FTLD or for MCI due to DLB. However, the high negative predictive value of FDG-PET and the existence of different disease-specific patterns of hypometabolism support the consensus recommendations for the clinical use of this imaging technique in MCI subjects.
Conclusions
FDG-PET has clinical utility on a fair level of evidence in detecting MCI due to AD. Although promising also in detecting MCI due to FTLD and MCI due to DLB, more research is needed to ultimately judge the clinical utility of FDG-PET in these entities.


Similar content being viewed by others
References
Molinuevo JL, Berthier ML, Rami L. Donepezil provides greater benefits in mild compared to moderate Alzheimer’s disease: implications for early diagnosis and treatment. Arch Gerontol Geriatr. 2011;52:18–22. https://doi.org/10.1016/j.archger.2009.11.004.
Bennett DA, Wilson RS, Boyle PA, Buchman AS, Schneider JA. Relation of neuropathology to cognition in persons without cognitive impairment. Ann Neurol. 2012;72:599–609. https://doi.org/10.1002/ana.23654.
Rowe CC, Ellis KA, Rimajova M, Bourgeat P, Pike KE, Jones G, et al. Amyloid imaging results from the Australian imaging, biomarkers and lifestyle (AIBL) study of aging. Neurobiol Aging. 2010;31:1275–83. https://doi.org/10.1016/j.neurobiolaging.2010.04.007.
Frisoni GB, Boccardi M, Barkhof F, Blennow K, Cappa S, Chiotis K, et al. Strategic roadmap for an early diagnosis of Alzheimer’s disease based on biomarkers. Lancet Neurol. 2017;16:661–76. https://doi.org/10.1016/S1474-4422(17)30159-X.
Borroni B, Cosseddu M, Pilotto A, Premi E, Archetti S, Gasparotti R, et al. Early stage of behavioral variant frontotemporal dementia: clinical and neuroimaging correlates. Neurobiol Aging. 2015;36:3108–15. https://doi.org/10.1016/j.neurobiolaging.2015.07.019.
Rogalski EJ, Mesulam MM. Clinical trajectories and biological features of primary progressive aphasia (PPA). Curr Alzheimer Res. 2009;6:331–6.
Boccardi M, Festari C, Altomare D, Gandolfo F, Orini S, Nobili F, et al. Assessing FDG-PET diagnostic accuracy studies to develop recommendations for clinical use in dementia. Eur J Nucl Med Mol Imaging. 2018. https://doi.org/10.1007/s00259-018-4024-1.
Leone MA, Brainin M, Boon P, Pugliatti M, Keindl M, Bassetti CL. Guidance for the preparation of neurological management guidelines by EFNS scientific task forces - revised recommendations 2012. Eur J Neurol. 2013;20:410–9. https://doi.org/10.1111/ene.12043.
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62:1006–12. https://doi.org/10.1016/j.jclinepi.2009.06.005.
McKhann GM, Jack CR, Albert MS, Knopman DS, Sperling RA, Carrillo MC, et al. Introduction to the recommendations from the National Institute on Aging-Alzheimer’s association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:257–62. https://doi.org/10.1016/j.jalz.2011.03.004.
Dubois B, Feldman HH, Jacova C, Cummings JL, Dekosky ST, Barberger-Gateau P, et al. Revising the definition of Alzheimer’s disease: a new lexicon. Lancet Neurol. 2010;9:1118–27. https://doi.org/10.1016/S1474-4422(10)70223-4.
Dubois B, Hampel H, Feldman HH, Scheltens P, Aisen P, Andrieu S, et al. Preclinical Alzheimer’s disease: definition, natural history, and diagnostic criteria. Alzheimers Dement. 2016;12:292–323. https://doi.org/10.1016/j.jalz.2016.02.002.
Rascovsky K, Hodges JR, Knopman D, Mendez MF, Kramer JH, Neuhaus J, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134:2456–77. https://doi.org/10.1093/brain/awr179.
Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology. 1998;51:1546–54.
Mosconi L, Tsui WH, Herholz K, Pupi A, Drzezga A, Lucignani G, et al. Multicenter standardized 18F-FDG PET diagnosis of mild cognitive impairment, Alzheimer’s disease, and other dementias. J Nucl Med. 2008;49:390–8. https://doi.org/10.2967/jnumed.107.045385.
Lowe VJ, Kemp BJ, Jack CR, Senjem M, Weigand S, Shiung M, et al. Comparison of 18F-FDG and PiB PET in cognitive impairment. J Nucl Med. 2009;50:878–86. https://doi.org/10.2967/jnumed.108.058529.
Langbaum JBS, Chen K, Lee W, Reschke C, Bandy D, Fleisher AS, et al. Categorical and correlational analyses of baseline fluorodeoxyglucose positron emission tomography images from the Alzheimer’s Disease Neuroimaging Initiative (ADNI). NeuroImage. 2009;45:1107–16. https://doi.org/10.1016/j.neuroimage.2008.12.072.
Caroli A, Prestia A, Chen K, Ayutyanont N, Landau SM, Madison CM, et al. Summary metrics to assess Alzheimer disease-related Hypometabolic pattern with 18F-FDG PET: head-to-head comparison. J Nucl Med. 2012;53:592–600. https://doi.org/10.2967/jnumed.111.094946.
Kim SH, Seo SW, Yoon DS, Chin J, Lee BH, Cheong HK, et al. Comparison of neuropsychological and fdg-pet findings between early- versus late-onset mild cognitive impairment: a five-year longitudinal study. Dement Geriatr Cogn Disord. 2010;29:213–23. https://doi.org/10.1159/000278422.
Mosconi L, Tsui WH, Pupi A, De Santi S, Drzezga A, Minoshima S, et al. (18)F-FDG PET database of longitudinally confirmed healthy elderly individuals improves detection of mild cognitive impairment and Alzheimer’s disease. J Nucl Med. 2007;48:1129–34. https://doi.org/10.2967/jnumed.107.040675.
Habeck C, Risacher S, Lee GJ, Glymour MM, Mormino E, Mukherjee S, et al. Relationship between baseline brain metabolism measured using [18F]FDG PET and memory and executive function in prodromal and early Alzheimer’s disease. Brain Imaging Behav. 2012;6:568–83. https://doi.org/10.1007/s11682-012-9208-x.
Landau SM, Harvey D, Madison CM, Koeppe RA, Reiman EM, Foster NL, et al. Associations between cognitive, functional, and FDG-PET measures of decline in AD and MCI. Neurobiol Aging. 2011;32:1207–18. https://doi.org/10.1016/j.neurobiolaging.2009.07.002.
Nobili F, Mazzei D, Dessi B, Morbelli S, Brugnolo A, Barbieri P, et al. Unawareness of memory deficit in amnestic MCI: FDG-PET findings. J Alzheimers Dis. 2010;22:993–1003. https://doi.org/10.3233/JAD-2010-100423.
Pagani M, De Carli F, Morbelli S, Öberg J, Chincarini A, Frisoni GB, et al. Volume of interest-based [18F]fluorodeoxyglucose PET discriminates MCI converting to Alzheimer’s disease from healthy controls. A European Alzheimer’s disease consortium (EADC) study. NeuroImage Clin. 2015;7:34–42. https://doi.org/10.1016/j.nicl.2014.11.007.
Prestia A, Caroli A, van der Flier WM, Ossenkoppele R, Van Berckel B, Barkhof F, et al. Prediction of dementia in MCI patients based on core diagnostic markers for Alzheimer disease. Neurology. 2013;80:1048–56. https://doi.org/10.1212/WNL.0b013e3182872830.
Zhang S, Han D, Tan X, Feng J, Guo Y, Ding Y. Diagnostic accuracy of 18F-FDG and 11C-PIB-PET for prediction of short-term conversion to Alzheimer’s disease in subjects with mild cognitive impairment. Int J Clin Pract. 2012;66:185–98. https://doi.org/10.1111/j.1742-1241.2011.02845.x.
Yuan Y, Gu Z-X, Wei W-S. Fluorodeoxyglucose-positron-emission tomography, single-photon emission tomography, and structural MR imaging for prediction of rapid conversion to Alzheimer disease in patients with mild cognitive impairment: a meta-analysis. AJNR Am J Neuroradiol. 2009;30:404–10. https://doi.org/10.3174/ajnr.A1357.
Frisoni GB, Bocchetta M, Chételat G, Rabinovici GD, De Leon MJ, Kaye J, et al. Imaging markers for Alzheimer disease: which vs how. Neurology. 2013;81:487–500. https://doi.org/10.1212/WNL.0b013e31829d86e8.
Drzezga A, Lautenschlager N, Siebner H, Riemenschneider M, Willoch F, Minoshima S, et al. Cerebral metabolic changes accompanying conversion of mild cognitive impairment into alzheimer’s disease: a PET follow-up study. Eur J Nucl Med Mol Imaging. 2003;30:1104–13. https://doi.org/10.1007/s00259-003-1194-1.
Chen K, Ayutyanont N, Langbaum JBS, Fleisher AS, Reschke C, Lee W, et al. Characterizing Alzheimer’s disease using a hypometabolic convergence index. NeuroImage. 2011;56:52–60. https://doi.org/10.1016/j.neuroimage.2011.01.049.
Garibotto V, Borroni B, Kalbe E, Herholz K, Salmon E, Holtoff V, et al. Education and occupation as proxies for reserve in aMCI converters and AD: FDG-PET evidence. Neurology. 2008;71:1342–9. https://doi.org/10.1212/01.wnl.0000327670.62378.c0.
Morbelli S, Piccardo A, Villavecchia G, Dessi B, Brugnolo A, Piccini A, et al. Mapping brain morphological and functional conversion patterns in amnestic MCI: a voxel-based MRI and FDG-PET study. Eur J Nucl Med Mol Imaging. 2010;37:36–45. https://doi.org/10.1007/s00259-009-1218-6.
Morbelli S, Drzezga A, Perneczky R, Frisoni GB, Caroli A, van Berckel BNM, et al. Resting metabolic connectivity in prodromal Alzheimer’s disease. A European Alzheimer disease consortium (EADC) project. Neurobiol Aging. 2012;33:2533–50. https://doi.org/10.1016/j.neurobiolaging.2012.01.005.
Nobili F, Salmaso D, Morbelli S, Girtler N, Piccardo A, Brugnolo A, et al. Principal component analysis of FDG PET in amnestic MCI. Eur J Nucl Med Mol Imaging. 2008;35:2191–202. https://doi.org/10.1007/s00259-008-0869-z.
Pagani M, Dessi B, Morbelli S, Brugnolo A, Salmaso D, Piccini A, et al. MCI patients declining and not-declining at mid-term follow-up: FDG-PET findings. Curr Alzheimer Res. 2010;7:287–94.
Landau SM, Mintun MA, Joshi AD, Koeppe RA, Petersen RC, Aisen PS, et al. Amyloid deposition, hypometabolism, and longitudinal cognitive decline. Ann Neurol. 2012;72:578–86. https://doi.org/10.1002/ana.23650.
Arbizu J, Prieto E, Martínez-Lage P, Martí-Climent JM, García-Granero M, Lamet I, et al. Automated analysis of FDG PET as a tool for single-subject probabilistic prediction and detection of Alzheimer’s disease dementia. Eur J Nucl Med Mol Imaging. 2013;40:1394–405. https://doi.org/10.1007/s00259-013-2458-z.
Cabral C, Morgado PM, Campos Costa D, Silveira M. Predicting conversion from MCI to AD with FDG-PET brain images at different prodromal stages. Comput Biol Med. 2015;58:101–9. https://doi.org/10.1016/j.compbiomed.2015.01.003.
Chetelat G, Desgranges B, de la Sayette V, Viader F, Eustache F, Baron J-C. Mild cognitive impairment: can FDG-PET predict who is to rapidly convert to Alzheimer’s disease? Neurology. 2003;60:1374–7. https://doi.org/10.1212/01.WNL.0000055847.17752.E6.
Drzezga A, Grimmer T, Riemenschneider M, Lautenschlager N, Siebner H, Alexopoulus P, et al. Prediction of individual clinical outcome in MCI by means of genetic assessment and (18)F-FDG PET. J Nucl Med. 2005;46:1625–32.
Gray KR, Wolz R, Heckemann RA, Aljabar P, Hammers A, Rueckert D. Multi-region analysis of longitudinal FDG-PET for the classification of Alzheimer’s disease. NeuroImage. 2012;60:221–9. https://doi.org/10.1016/j.neuroimage.2011.12.071.
Herholz K, Westwood S, Haense C, Dunn G. Evaluation of a calibrated (18)F-FDG PET score as a biomarker for progression in Alzheimer disease and mild cognitive impairment. J Nucl Med. 2011;52:1218–26. https://doi.org/10.2967/jnumed.111.090902.
Ito K, Fukuyama H, Senda M, Ishii K, Maeda K, Yamamoto Y, et al. Prediction of outcomes in mild cognitive impairment by using 18F-FDG-PET: a multicenter study. J Alzheimers Dis. 2015;45:543–52. https://doi.org/10.3233/JAD-141338.
Morbelli S, Brugnolo A, Bossert I, Buschiazzo A, Frisoni GB, Galluzzi S, et al. Visual versus semi-quantitative analysis of 18F-FDG-PET in amnestic MCI: an European Alzheimer’s disease consortium (EADC) project. J Alzheimers Dis. 2015;44:815–26. https://doi.org/10.3233/JAD-142229.
Mosconi L, Perani D, Sorbi S, Herholz K, Nacmias B, Holthoff V, et al. MCI conversion to dementia and the APOE genotype: a prediction study with FDG-PET. Neurology. 2004;63:2332–40. https://doi.org/10.1212/01.WNL.0000147469.18313.3B.
Toussaint P-J, Perlbarg V, Bellec P, Desarnaud S, Lacomblez L, Doyon J, et al. Resting state FDG-PET functional connectivity as an early biomarker of Alzheimer’s disease using conjoint univariate and independent component analyses. NeuroImage. 2012;63:936–46. https://doi.org/10.1016/j.neuroimage.2012.03.091.
Young J, Modat M, Cardoso MJ, Mendelson A, Cash D, Ourselin S. Accurate multimodal probabilistic prediction of conversion to Alzheimer’s disease in patients with mild cognitive impairment. NeuroImage Clin. 2013;2:735–45. https://doi.org/10.1016/j.nicl.2013.05.004.
Choo IH, Ni R, Schöll M, Wall A, Almkvist O, Nordberg A. Combination of 18F-FDG PET and cerebrospinal fluid biomarkers as a better predictor of the progression to Alzheimer’s disease in mild cognitive impairment patients. J Alzheimers Dis. 2013;33:929–39. https://doi.org/10.3233/JAD-2012-121489.
Landau SM, Harvey D, Madison CM, Reiman EM, Foster NL, Aisen PS, et al. Comparing predictors of conversion and decline in mild cognitive impairment. Neurology. 2010;75:230–8. https://doi.org/10.1212/WNL.0b013e3181e8e8b8.
Poljansky S, Ibach B, Hirschberger B, Männer P, Klünemann H, Hajak G, et al. A visual [18F]FDG-PET rating scale for the differential diagnosis of frontotemporal lobar degeneration. Eur Arch Psychiatry Clin Neurosci. 2011;261:433–46. https://doi.org/10.1007/s00406-010-0184-0.
Banzo I, Jiménez-Bonilla J, Ortega-Nava F, Quirce R, Martínez-Rodríguez I, de Arcocha-Torres M, et al. Amyloid imaging with 11C-PIB PET/CT and glucose metabolism with 18F-FDG PET/CT in a study on cognitive impairment in the clinical setting. Nucl Med Commun. 2014;35:238–44. https://doi.org/10.1097/MNM.0000000000000042.
Bergeron D, Beauregard J-M, Guimond J, Fortin M-P, Houde M, Poulin S, et al. Clinical impact of a second FDG-PET in atypical/unclear dementia syndromes. J Alzheimers Dis. 2016;49:695–705. https://doi.org/10.3233/JAD-150302.
Morbelli S, Ferrara M, Fiz F, Dessi B, Arnaldi D, Picco A, et al. Mapping brain morphological and functional conversion patterns in predementia late-onset bvFTD. Eur J Nucl Med Mol Imaging. 2016;43:1337–47. https://doi.org/10.1007/s00259-016-3335-3.
Döbert N, Pantel J, Frölich L, Hamscho N, Menzel C, Grünwald F. Diagnostic value of FDG-PET and HMPAO-SPET in patients with mild dementia and mild cognitive impairment: metabolic index and perfusion index. Dement Geriatr Cogn Disord. 2005;20:63–70. https://doi.org/10.1159/000085857.
Perani D, Della Rosa PA, Cerami C, Gallivanone F, Fallanca F, Vanoli EG, et al. Validation of an optimized SPM procedure for FDG-PET in dementia diagnosis in a clinical setting. NeuroImage Clin. 2014;6:445–54. https://doi.org/10.1016/j.nicl.2014.10.009.
Grimmer T, Wutz C, Alexopoulos P, Drzezga A, Förster S, Förstl H, et al. Visual versus fully automated analyses of 18F-FDG and amyloid PET for prediction of dementia due to Alzheimer disease in mild cognitive impairment. J Nucl Med. 2016;57:204–7. https://doi.org/10.2967/jnumed.115.163717.
Cerami C, Della Rosa PA, Magnani G, Santangelo R, Marcone A, Cappa SF, et al. Brain metabolic maps in mild cognitive impairment predict heterogeneity of progression to dementia. NeuroImage Clin. 2015;7:187–94. https://doi.org/10.1016/j.nicl.2014.12.004.
Fujishiro H, Iseki E, Kasanuki K, Murayama N, Ota K, Suzuki M, et al. Glucose hypometabolism in primary visual cortex is commonly associated with clinical features of dementia with Lewy bodies regardless of cognitive conditions. Int J Geriatr Psychiatry. 2012;27:1138–46. https://doi.org/10.1002/gps.2836.
Perani D, Cerami C, Caminiti SP, Santangelo R, Coppi E, Ferrari L, et al. Cross-validation of biomarkers for the early differential diagnosis and prognosis of dementia in a clinical setting. Eur J Nucl Med Mol Imaging. 2016;43:499–508. https://doi.org/10.1007/s00259-015-3170-y.
Fujishiro H, Iseki E, Murayama N, Yamamoto R, Higashi S, Kasanuki K, et al. Diffuse occipital hypometabolism on [18 F]-FDG PET scans in patients with idiopathic REM sleep behavior disorder: prodromal dementia with Lewy bodies? Psychogeriatrics. 2010;10:144–52. https://doi.org/10.1111/j.1479-8301.2010.00325.x.
Fujishiro H, Iseki E, Kasanuki K, Chiba Y, Ota K, Murayama N, et al. A follow up study of non-demented patients with primary visual cortical hypometabolism: prodromal dementia with Lewy bodies. J Neurol Sci. 2013;334:48–54. https://doi.org/10.1016/j.jns.2013.07.013.
Pardo JV, Lee JT, Kuskowski MA, Munch KR, Carlis JV, Sheikh SA, et al. Fluorodeoxyglucose positron emission tomography of mild cognitive impairment with clinical follow-up at 3 years. Alzheimers Dement. 2010;6:326–33. https://doi.org/10.1016/j.jalz.2009.09.005.
Clerici F, Del Sole A, Chiti A, Maggiore L, Lecchi M, Pomati S, et al. Differences in hippocampal metabolism between amnestic and non-amnestic MCI subjects: automated FDG-PET image analysis. Q J Nucl Med Mol Imaging. 2009;53:646–57.
Mosconi L, Mistur R, Switalski R, Tsui WH, Glodzik L, Li Y, et al. FDG-PET changes in brain glucose metabolism from normal cognition to pathologically verified Alzheimer’s disease. Eur J Nucl Med Mol Imaging. 2009;36:811–22. https://doi.org/10.1007/s00259-008-1039-z.
Perani D, Daniela P, Schillaci O, Orazio S, Padovani A, Alessandro P, et al. A survey of FDG- and amyloid-PET imaging in dementia and GRADE analysis. Biomed Res Int. 2014;2014:785039. https://doi.org/10.1155/2014/785039.
Smailagic N, Vacante M, Hyde C, Martin S, Ukoumunne O, Sachpekidis C. 18 F-FDG PET for the early diagnosis of Alzheimer’s disease dementia and other dementias in people with mild cognitive impairment (MCI). Cochrane Database Syst Rev. 2015;1:CD010632. https://doi.org/10.1002/14651858.CD010632.pub2.
Garibotto V, Herholz K, Boccardi M, Picco A, Varrone A, Nordberg A, et al. Clinical validity of brain fluorodeoxyglucose positron emission tomography as a biomarker for Alzheimer’s disease in the context of a structured 5-phase development framework. Neurobiol Aging. 2017;52:183–95. https://doi.org/10.1016/j.neurobiolaging.2016.03.033.
Dubois B, Feldman HH, Jacova C, Hampel H, Molinuevo JL, Blennow K, et al. Advancing research diagnostic criteria for Alzheimer’s disease: the IWG-2 criteria. Lancet Neurol. 2014;13:614–29. https://doi.org/10.1016/S1474-4422(14)70090-0.
Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:270–9. https://doi.org/10.1016/j.jalz.2011.03.008.
Gossink FT, Dols A, Kerssens CJ, Krudop WA, Kerklaan BJ, Scheltens P, et al. Psychiatric diagnoses underlying the phenocopy syndrome of behavioural variant frontotemporal dementia. J Neurol Neurosurg Psychiatry. 2016;87:64–8. https://doi.org/10.1136/jnnp-2014-308284.
Kipps CM, Hodges JR, Fryer TD, Nestor PJ. Combined magnetic resonance imaging and positron emission tomography brain imaging in behavioural variant frontotemporal degeneration: refining the clinical phenotype. Brain. 2009;132:2566–78. https://doi.org/10.1093/brain/awp077.
Nestor P, Altomare D, Festari C, Drzezga A, Rivolta J, Walker Z, et al. Clinical utility of FDG-PET for the differential diagnosis among the main forms of dementia. Eur J Nucl Med Mol Imaging. 2018. https://doi.org/10.1007/s00259-018-4035-y.
Nobili F, Festari C, Altomare D, Agosta F, Orini S, Van Laere K, et al. Automated assessment of FDG-PET for the differential diagnosis in patients with neurodegenerative disorders. Eur J Nucl Med Mol Imaging. 2018. https://doi.org/10.1007/s00259-018-4030-3.
Acknowledgements
The procedure for assessing scientific evidence and defining consensual recommendations was funded by the EANM and by the EAN. We thank the Guidelines Working Group of the EAN, particularly Simona Arcuti and Maurizio Leone, for their methodological advice.
Funding
This project was partially funded by the European Association of Nuclear Medicine (EANM) and the European Academy of Neurology (EAN).
Author information
Authors and Affiliations
Consortia
Corresponding authors
Ethics declarations
Conflict of interest
Javier Arbizu: received grants from Eli-Lilly & Co, Piramal and GE Healthcare.
Cristina Festari: declares that she has no conflict of interest.
Daniele Altomare was the recipient of the grant allocated by the European Academy of Neurology (EAN) for data extraction and evidence assessment for the present project.
Zuzana Walker: received from G.E. Healthcare grants and tracers, personal fees for consultancy and speakers fee.
Femke Bouwman: declares that she has no conflict of interest.
Jasmine Rivolta: declares that she has no conflict of interest.
Stefania Orini: declares that she has no conflict of interest.
Henrik Barthel: declares that he has no conflict of interest.
Federica Agosta: is Section Editor of NeuroImage: Clinical; has received speaker fees from Biogen Idec, Novartis, and Excellence in Medical Education; and receives or has received research supports from the Italian Ministry of Health, AriSLA (Fondazione Italiana di Ricerca per la SLA), and the European Research Council. She received personal fees from Elsevier Inc.
Alexander Drzezga: received grants and non-financial support from Eli-Lilly & Co, Siemens and GE Healthcare; he also received non-financial support from Piramal.
Peter Nestor: received radiotracer from Piramal at a discounted rate as part of a research collaboration.
Marina Boccardi has received funds from the European Association of Nuclear Medicine (EANM) to perform the evidence assessment and the global coordination of the present project. Moreover, she has received research grants from Piramal and served as a paid member of advisory boards for Eli Lilly.
Giovanni B Frisoni is principal investigator of industry-sponsored trials funded by AbbVie, Acadia, Altoida, Amoneta, Araclon, Biogen, Janssen, Novartis, Piramal; has received funding for investigator-initiated trials from GE, Piramal, and Avid-Lilly; and has received speaker fees from a number of pharma and imaging companies.
Flavio Nobili: received personal fees and non-financial support from GE Healthcare, non-financial support from Eli-Lilly and grants from Chiesi Farmaceutici.
Ethical approval
This is a review article that does not contain any original study with human participants performed by any of the authors. Ethical approval is shown in each of the quoted original papers.
Informed consent
not applicable, this is a review article. Informed consent statement is declared in each of the revised papers.
Rights and permissions
About this article
Cite this article
Arbizu, J., Festari, C., Altomare, D. et al. Clinical utility of FDG-PET for the clinical diagnosis in MCI. Eur J Nucl Med Mol Imaging 45, 1497–1508 (2018). https://doi.org/10.1007/s00259-018-4039-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-018-4039-7