Abstract
Objective
During neoadjuvant chemoradiotherapy for oesophageal cancer, or in the interval prior to surgery, some patients develop systemic metastasis. This study aimed to evaluate the diagnostic performance of 18F-FDG PET/CT for the detection of interval metastasis and to identify predictors of interval metastases in a large cohort of oesophageal cancer patients.
Methods
In total, 783 consecutive patients with potentially resectable oesophageal cancer who underwent chemoradiotherapy and pre- and post-treatment 18F-FDG PET/CT between 2006 and 2015 were analyzed from a prospectively maintained database. Diagnostic accuracy measures were calculated on a per-patient basis using histological verification or clinical follow-up as a reference standard. Multivariable logistic regression analysis was performed to determine pre-treatment predictors of interval metastasis. A prediction score was developed to predict the probability of interval metastasis.
Results
Of 783 patients that underwent 18F-FDG PET/CT restaging, 65 (8.3%) were found to have interval metastasis and 44 (5.6%) were deemed to have false positive lesions. The resulting sensitivity and specificity was 74.7% (95% CI: 64.3–83.4%) and 93.7% (95% CI: 91.6–95.4%), respectively. Multivariable analysis revealed that tumor length, cN status, squamous cell tumor histology, and baseline SUVmax were associated with interval metastasis. Based on these criteria, a prediction score was developed with an optimism adjusted C-index of 0.67 that demonstrated accurate calibration.
Conclusions
18F-FDG PET/CT restaging detects distant interval metastases in 8.3% of patients after chemoradiotherapy for oesophageal cancer. The provided prediction score may stratify risk of developing interval metastasis, and could be used to prioritize additional restaging modalities for patients most likely to benefit.
Similar content being viewed by others
Introduction
Oesophageal cancer affects more than 450,000 people annually, and is the sixth leading cause of cancer-related mortality worldwide [1]. Currently, surgical resection of the esophagus preceded by neoadjuvant chemoradiotherapy is the standard of care for patients with non-metastasized oesophageal cancer [1,2,3]. Definitive chemoradiotherapy is the preferred approach for inoperable locally advanced oesophageal cancer [4, 5]. In consequence of the duration of chemoradiotherapy and subsequent waiting time to surgery, systemic interval metastases may develop that were not visible during baseline staging [6,7,8]. In these patients curative treatment is no longer possible [9].
Currently, there is disagreement between guidelines as to whether all patients should be restaged after chemoradiotherapy [10,11,12]. In different international guidelines, routine restaging with computed tomography and integrated 18F-fluorodeoxyglucose positron emission tomography (18F-FDG PET/CT) is not recommended [11], or partially recommended for patients with cT3–4 or cN1–3 tumors [12]. To the contrary, the National Comprehensive Cancer Network advises restaging for all patients who receive preoperative chemoradiotherapy [10]. At present, little is known about which patients are at risk of developing interval metastases.
Several small studies have assessed the role of 18F-FDG PET/CT for pre-surgical restaging after neoadjuvant therapy, with reported incidence rates of interval metastases varying from 2% up to 26% [13, 14]. However, most studies did not report diagnostic accuracy measures (i.e. sensitivity, specificity) and included only a small number of patients [6,7,8]. Also, studies that have assessed clinical predictors for interval metastases are scarce [15]. Accurate prediction of disease progression during and shortly after chemoradiotherapy would enable surveillance tailored to each patient’s underlying risk of developing systemic disease.
The aim of the current study was two-fold. First, to quantify the incidence of interval metastases after chemoradiotherapy and evaluate the diagnostic performance of 18F-FDG PET/CT for the detection of interval metastases in a large cohort of patients. Second, to identify pre-treatment clinical predictors for interval metastases.
Methods
This retrospective study was approved by the institutional review board of the MD Anderson Cancer Center and the requirement to obtain informed consent was waived. The study was conducted in accordance with the Health Insurance Portability and Accountability Act (HIPAA), the checklist from the Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis statement (http://www.tripod-statement.org) [16], and the checklist from the STAndards for the Reporting of Diagnostic accuracy studies (STARD) statement (http://www.stard-statement.org) [17].
Study population
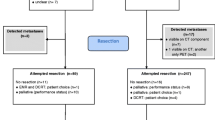
Data from consecutive patients with biopsy-proven adenocarcinoma or squamous cell carcinoma of the esophagus who received chemoradiotherapy (with or without surgery) at the University of Texas MD Anderson Cancer Center from January 2006 to July 2013 were extracted from a prospective collected departmental registry. Inclusion criteria were; non-metastatic potentially resectable oesophageal cancer (cT1N + M0 or cT2-4aM0 with nodes in the anatomic region of a 2-field lymph node dissection), scheduled radiation dose of 45 or 50.4 Gy with concurrent chemotherapy, staging with 18F-FDG PET/CT before and after chemoradiotherapy. Patients were excluded if the time interval between completion of chemoradiotherapy and 18F-FDG PET/CT restaging was more than 3 months. The flow of patient selection is summarized in Fig. 1. Disease was staged in accordance with the 7th edition of the International Union Against Cancer for cTNM-classification [18]. Initial diagnostic work-up included endoscopy with biopsy, endoscopic ultrasound (including fine-needle aspiration if indicated), and 18F-FDG PET/CT.
Treatment protocol
Chemoradiotherapy treatment generally consisted of a fluoropyrimidine (IV or oral) with either platinum- or taxane-based chemotherapy with concurrent radiotherapy (45 or 50.4 Gy in fractions of 1.8 Gy) (Table 1). Four to 6 weeks after completion of chemoradiotherapy, all patients underwent re-staging procedures and were discussed in multidisciplinary tumor conferences. Patients that were deemed eligible for surgical treatment proceeded to oesophagectomy. Surgical treatment consisted of transthoracic oesophagectomy combined with lymphadenectomy.
Image acquisition and analysis
Patients were scanned before and after completion of chemoradiotherapy on a dedicated PET/CT system (Discovery RX, ST, or STE; GE Medical Systems, Milwaukee [WI], USA). After fasting for at least 6 h, patients were injected with 18F-FDG (555–740 MBq). An unenhanced CT was acquired for attenuation correction purposes (120 kV peaks, 300 mA, 0.5 s rotation, pitch of 1.375, slice thickness 3.75 mm, and slice interval 3.27 mm). PET scans were acquired 60–90 min after administration of 18F-FDG in either two-dimensional (2D) or three-dimensional (3D) acquisition mode.
PET/CT interpretations rendered as part of the clinical care were extracted from the original PET/CT reports. All 18F-FDG PET/CT images were reviewed by experienced nuclear medicine radiologists who were aware of patients’ information and previous clinical findings. The images were evaluated for the presence of new lesions with non-physiological 18F-FDG accumulation. Suspicious lesions on CT scans with increased focal 18F-FDG uptake were indicated as malignant. 18F-FDG PET/CT images were interpreted as positive for interval metastasis when new malignant lesions were found outside the anatomic dissection plane of an oesophagectomy combined with a two-field lymphadenectomy.
Reference standard
A composite reference standard combining histologic proof and/or imaging follow-up was used to confirm the disease status of patients after restaging with 18F-FDG PET/CT. Histologically verified PET/CT-positive lesions or lesions showing an increase in size or 18F-FDG uptake on subsequent radiological follow-up were considered as true-positive (TP). Clinical follow-up was used as reference standard for patients with a negative 18F-FDG PET/CT during restaging. Patients were followed every 3 months during the first year after treatment, which included physical examination, blood tests, and 18F-FDG PET/CT scans. The restaging 18F-FDG PET/CT was considered false negative (FN) in case patients developed new metastatic disease within 3 months after the initial restaging 18F-FDG PET/CT scan. Patients without confirmed systemic disease progression during follow-up were considered as true-negative (TN).
Pre-treatment predictors
All patient, tumor, and treatment-related characteristics as reported in Table 1 were derived from the prospective collected departmental registry. Initial selection of predictors for interval metastasis detected by 18F-FDG PET/CT restaging were pre-specified based on previous literature to prevent overfitting of the model. Categories were based on previously published cut-off points or estimated by receiver operating characteristic (ROC) curve analysis while maximizing sensitivity and specificity. Clinical factors available before initiation of treatment that have previously been identified as prognostic factors in oesophageal cancer included gender [19], age (dichotomized into <65 and ≥ 65) [20], Histology (adenocarcinoma versus squamous cell carcinoma [3, 20], histologic differentiation grade (good/moderate versus poor) [20, 21], signet ring cell adenocarcinoma [22, 23], EUS-based tumor length (dichotomized into <4.0 cm and ≥ 4.0 cm) [24, 25], nontraversability by EUS [15, 24], tumor location (upper/middle versus distal or gastro-oesophageal junction) [18], clinical T-status (T1b-2 versus T3–4) [19, 20], clinical N status (N0 versus N1–3) [20, 21], maximum lymph node diameter measured on axial CT image (<1.0 cm versus ≥1 cm) [26, 27], and 18F-FDG avid nodes at baseline PET [15]. The maximum standardized uptake value (SUVmax) of the primary tumor was dichotomized into <9.6 and ≥ 9.6 based on ROC curve analysis.
Statistics
Patient and treatment-related characteristics were described as frequencies with percentages for categorical variables, mean with standard deviation (SD) for normally distributed variables and median with range for skewed distributions. Sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV) and accuracy of 18F-FDG PET/CT for the detection of interval metastasis were calculated with 95% confidence interval (CI) on a per-patient basis. Kaplan-Meier curves were used to assess overall survival, and survival differences were evaluated using the log-rank test. Statistical analysis was performed using SPSS version 24.0 (IBM Corp., Armonk, NY) and R 3.1.2 open-source software (http://www.R-project.org, ‘rms’ package). A p-value of <0.05 was considered statistically significant.
Model development
The association between clinical characteristics and interval metastasis was studied using the chi-square test. All potential prespecified predictors for interval metastasis were included in a multivariable logistic regression model. The initial logistic regression model was reduced using backward stepwise elimination based on Akaike Information Criteria. The discriminative ability of the final model was evaluated using receiver operating characteristics curve analysis providing the concordance statistic (C-statistic). For internal validation the model was subjected to 200 bootstrap resamples to calculate the optimism of the model and the shrinkage factor, after which the C-statistic and the β-coefficients were adjusted. A practical scoring system was developed using the beta-regression coefficients of the predictors that remained in the final model. Calibration of the model was evaluated by plotting the mean predicted probability of interval metastasis versus the observed percentage of interval metastasis for each level of the prediction score.
Results
In the study period, a total of 783 patients diagnosed with oesophageal cancer who met our inclusion and exclusion criteria underwent chemoradiotherapy followed by a restaging 18F-FDG PET/CT scan (Fig. 1). The distribution of patient, tumor, and treatment-related characteristics are summarized in Table 1. The study population had a mean age of 62.5 years (SD: 10.6 years), and the majority of patients were male (86.2%). The predominant histologic tumor type was adenocarcinoma (85.8%), and the most common clinical tumor stage was cT3 (87.1%). The mean time interval between completion of chemoradiotherapy and 18F-FDG PET/CT restaging was 41.3 days (SD: 10.7). After completion of chemoradiotherapy, 450 (57.5%) patients underwent oesophageal resection.
Diagnostic accuracy
In 109 of 783 (13.9%) patients, new potential metastatic lesions were detected during PET/CT restaging. Of these patients, 65 (TP: 65/783; 8.3%) were confirmed to have true metastatic disease by histology (n = 21) or clinical follow-up (n = 44), and 44 were deemed to have false positive results (FP: 44/783; 5.6%) (see Figs. 2 and 3 for examples). The location and treatment of new metastatic lesions are presented in Table 2, and the location of false-positives in Fig. 1. Median overall survival of patients with interval metastasis was 6 months (95% CI: 4–8 months), compared to 59 months for patients without metastatic disease at restaging (47–70 months).
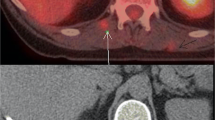
Examples of true positive metastatic lesions detected by 18F-FDG PET/CT restaging. (a/c): 80-year-old woman with adenocarcinoma of the distal esophagus treated with chemoradiation. The maximum intensity projection PET image shows multiple hypermetabolic foci of the liver and multiple soft tissue lesions that were confirmed malignant with follow-up scans. (b): 65-year-old male with squamous cell carcinoma of the distal esophagus who had undergone chemoradiotherapy. The PET/CT image showed 18F-FDG accumulation in the liver and in the thoracic spine at T5. Follow-up CT showed disease progression
Examples of new non-malignant 18F-FDG avid lesions detected by 18F-FDG PET/CT restaging. (a): 78-year-old woman with squamous cell carcinoma of the esophagus treated with chemoradiation. The PET/CT image shows new opacities within the left lower lobe with corresponding areas of 18F-FDG activity. The new lesion was within the presumed radiation field (b) and the appearance was most compatible with radiation-induced pneumonitis (scan was regarded as ‘true negative’ for new metastatic disease). (c): 42-year-old female with adenocarcinoma of the distal esophagus who had undergone chemoradiotherapy. The PET/CT images show linear 18F-FDG accumulation within the lateral aspect of the left hepatic lobe. The new lesion was within the presumed radiation field (d) and was thought to be related to radiation therapy changes, which was confirmed with an MRI scan (scan was regarded as ‘false positive’ as additional imaging was required to exclude metastatic disease)
In patients with no evidence of interval metastasis after initial restaging with 18F-FDG PET/CT, new metastatic lesions were identified in 22 (FN: 22/783; 2.8%) patients within 3 months of follow-up (Fig. 1). The resulting overall per-patient sensitivity and specificity of 18F-FDG PET/CT to detect interval metastasis was 74.7% (95% CI: 64.3–83.4%) and 93.7% (95% CI: 91.6–95.4%), respectively. Positive and negative predictive values of PET/CT were 59.6% (95% CI: 52.0–66.9%) and 96.8% (95% CI: 95.4–97.7%), respectively (Table 3).
Pre-treatment prediction of interval metastasis
The univariable associations of clinical factors with interval metastasis after chemoradiotherapy are summarized in Table 1. After multivariable analysis, clinical nodal involvement (odds ratio [OR]: 2.91, 95% CI: 1.34–6.32), EUS-based tumor length ≥ 4 cm (OR: 2.68, 95% CI: 1.11–6.52), squamous cell tumor histology (OR: 1.65, 95% CI: 0.86–3.17) and baseline SUVmax ≥ 9.6 (OR: 1.66, 95% CI: 0.94–2.93) remained independently predictive for the occurrence of interval metastasis (Table 3). The discriminative ability of the final model was reasonable with an apparent C-statistic of 0.69, and 0.67 after adjustment for optimism. The shrinkage factor for the coefficients was 0.88. The adjusted β-coefficients of the prediction model for interval metastasis after shrinkage are presented in Table 3.
A practical prediction tool for the development of interval metastasis was developed based on the 4 predictors that remained in the final model. Based on the adjusted β-coefficients, each variable was converted into a corresponding number of points (multiplied by 2) rounded to its nearest integer. The total risk score was calculated by adding up the number of points obtained for each clinical predictor (cN status + EUS-based tumor length + tumor histology + baseline SUVmax = risk score; Table 4). In Table 4, also, the corresponding observed risk for interval metastasis can be found for the different risk scores. The correspondence between the predicted risk of interval metastasis by the risk score and actual observed interval metastasis indicated good calibration (Fig. 4). In patients without interval metastasis after 18F-FDG PET/CT restaging, the risk score was also significantly associated with survival (Fig. 5, p = 0.001).
Discussion
Findings in the current study demonstrate that 18F-FDG PET/CT restaging after neoadjuvant chemoradiotherapy detects interval metastases in 8% of oesophageal cancer patients, with a patient-based sensitivity and specificity of 75% and 94%, respectively. Independent risk factors for the development of interval metastases include clinical nodal involvement, EUS-based tumor length of ≥4 cm, squamous cell tumor histology, and baseline SUVmax of ≥9.6. Based on these findings, a prediction score was developed which may provide physicians a tool for objectively assessing the risk of interval metastasis in patients with oesophageal cancer.
Accurate preoperative detection of (interval) metastasis of oesophageal cancer is crucial for optimal selection of patients suitable for surgery. In patients with accurately detected interval metastasis, surgery is expected to provide no benefit in terms of survival, but rather to decrease quality of life due to highly morbid surgery with subsequent recovery time [28]. In this regard, the findings of the current study indicate that 8% of patients were spared a futile oesophagectomy as a result of our routine 18F-FDG PET/CT restaging protocol. Although previous studies on this topic have reported detection rates of interval metastasis ranging between 2% and 26%, guideline recommendations on restaging patients after neoadjuvant chemoradiotherapy for oesophageal cancer remain contradictory [10,11,12].
The incidence of interval metastasis in the current study is consistent with the results of previous reports [6,7,8]; however, there are some important other aspects of 18F-FDG PET/CT restaging that should be considered. Our findings indicate that in 86% of patients, no new lesion will be detected during restaging after chemoradiotherapy, indicating that limited impact on patient management is anticipated in the majority of patients. In another 6% of the patients, restaging results in false positive findings introducing unnecessary imaging and biopsy procedures. It should be noted that this work-up is associated with additional costs and that biopsy procedures are not without risks [29].
Consequently, a more individualized application of 18F-FDG PET/CT restaging could reduce the number of unbeneficial diagnostic tests. Yet, little is known about what patients are at risk for developing interval metastases, and the small number of patients in the previous mentioned studies precludes assessment of predictors for interval metastasis after neoadjuvant therapy [6,7,8, 15]. These findings encouraged us to develop a risk prediction score for interval metastases that may guide a more targeted application of 18F-FDG PET/CT restaging. This may especially be of interest for hospitals/regions with limited resources that have not yet implemented 18F-FDG PET/CT restaging in their routine clinical practice due to associated costs.
The proposed risk score - based on well-recognized prognostic factors [3, 20, 21, 24, 25, 30] - has reasonable predictive value and may guide clinical decision making. The data indicate that patients with low scores have limited risk of interval metastases, and that in these patients a restaging 18F-FDG PET/CT may be safely omitted without subjecting the patient to the risks of further diagnostic tests. Increasing the threshold for restaging patients with 18F-FDG PET/CT based on the proposed risk score will result in a further reduction of unnecessary additional scans and biopsies (false positives), but at the potential cost of missing interval metastasis (false negatives). Determining an appropriate threshold at which to initiate restaging will depend on patients’ and physicians’ judgments about the harm of missed interval metastasis versus unnecessary diagnostic tests and available resources.
The relatively high incidence (11%) of early disease progression already within 6 months after restaging (Fig. 1) suggests that small distant metastases, which are not detected by 18F-FDG PET/CT, may already have occurred at the time of restaging [31]. This indicates that while 18F-FDG PET/CT detects a substantial proportion of interval metastasis, it is sometimes insufficient to detect all early disease progression. Therefore, one may consider close monitoring of high-risk patients with additional (perioperative) restaging. This suggestion is supported by our finding that the risk score was also predictive for survival after initial restaging.
In the context of clinical decision-making with regard to those patients who are most likely to benefit from an oesophageal resection after chemoradiotherapy, the prediction of pathological response to neoadjuvant therapy may be another motivation to perform 18F-FDG PET/CT restaging. It has been suggested that preoperative identification of patients with a pathologic complete response – aided by information derived from 18F-FDG PET/CT - could enable a wait-and-see approach with omission of surgery [19, 32]. However, currently uncertainty continues to exist over the clinical benefit of 18F-FDG PET/CT with regard to the accuracy for differentiating between residual tumor and therapy induced inflammation after chemoradiotherapy [33,34,35]. Other reasons to perform a restaging scan include surgical planning (notable for GEJ tumors).
As discussed, the false positive rate of 6% during 18F-FDG PET/CT restaging was substantial, with the lungs and liver as the most frequent affected sites. This confirms previous findings in literature, with reported false positive rates ranging between 0% and 10% [6, 36] and liver and lung as the most commonly affected sites [29, 37]. This is likely caused by radiation-induced disease that may falsely indicate disease progression (Figs. 2 and 3). Previous studies evaluating new FDG-avid hepatic lesions within the presumed radiation field of patients with oesophageal cancer demonstrated that these lesions generally reflect radiation-induced liver disease rather than metastatic disease [37,38,39]. Evaluation of radiation fields may, therefore, aid in the assessment of restaging 18F-FDG PET/CT scans and further clinical decision-making [37].
Potential limitations of this study are that it used follow-up information as a reference standard, which is challenging because follow-up should be long enough to allow hidden cases of disease to progress to a detectable stage, while it should be short enough to prevent new cases that develop after restaging to be detected. Because the length of follow-up to determine disease-status is arbitrary, reported diagnostic accuracy measures may vary in cases of different follow-up lengths. Second, histological biopsy was not performed in all patients with suspected interval metastasis, which may have introduced reference test bias. Third, quantitative imaging values such as SUV may be biased by many factors related to clinical protocols and PET system settings, many of which are center or manufacturer dependent. Therefore, future studies that use quantitative imaging for prognostic modeling are encouraged to control biases through standardization of imaging procedures by using harmonization programs (e.g. Quantitative Imaging Biomarkers Alliance [40]). Furthermore, the current study represents a single-institution analysis where findings in general may not be generalizable to other centers. Therefore, external validation of the developed risk prediction score is recommended to determine generalizability [41].
Despite the aforementioned limitations, major strengths of this study include that it is the largest study so far to assess the diagnostic performance of 18F-FDG PET/CT for the detection of interval metastases. Furthermore, it provides the first clinically applicable risk prediction score for interval metastasis after chemoradiotherapy for oesophageal cancer.
Conclusion
18F-FDG PET/CT restaging detects true distant interval metastases in 8.3% of patients after chemoradiotherapy for oesophageal cancer. The provided prediction score stratifies risk of developing interval metastasis, and could be used to prioritize additional restaging modalities for patients most likely to benefit. Centers that do not routinely perform 18F-FDG PET/CT restaging, should at least consider scans for patients at high risk of interval metastases.
References
Pennathur A, Gibson MK, Jobe BA, Luketich JD. Oesophageal carcinoma. Lancet (London, England). 2013;381:400–12.
Sjoquist KM, Burmeister BH, Smithers BM, Zalcberg JR, Simes RJ, Barbour A, et al. Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: an updated meta-analysis. Lancet Oncol. 2011;12:681–92.
Shapiro J, van Lanschot JJB, Hulshof MCCM, van Hagen P, van Berge Henegouwen MI, Wijnhoven BPL, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol. 2015;16:1090–8.
Teoh AYB, Chiu PWY, Yeung WK, Liu SYW, Wong SKH, Ng EKW. Long-term survival outcomes after definitive chemoradiation versus surgery in patients with resectable squamous carcinoma of the esophagus: results from a randomized controlled trial. Ann Oncol. 2013;24:165–71.
Gwynne S, Hurt C, Evans M, Holden C, Vout L, Crosby T. Definitive chemoradiation for oesophageal cancer — a standard of care in patients with non-metastatic oesophageal cancer. Clin Oncol. 2011;23:182–8.
Blom RL, Schreurs WM, Belgers HJ, Oostenbrug LE, Vliegen RF, Sosef MN. The value of post-neoadjuvant therapy PET-CT in the detection of interval metastases in esophageal carcinoma. Eur J Surg Oncol. 2011;37:774–8.
Bruzzi JF, Munden RF, Truong MT, Marom EM, Sabloff BS, Gladish GW, et al. PET/CT of esophageal cancer: its role in clinical management 2007;27:1635–52.
Stiekema J, Vermeulen D, Vegt E, Voncken FE, Aleman BM, Sanders J, et al. Detecting interval metastases and response assessment using 18F-FDG PET/CT after neoadjuvant chemoradiotherapy for esophageal cancer. Clin Nucl Med. 2014;39:862–7.
Lagergren J, Smyth E, Cunningham D, Lagergren P. Oesophageal cancer. Lancet. 2017;390:2383–96.
NCCN Clinical practice guidelines in oncology (NCCN guidelines)—esophageal and Esophagogastric junction cancers version 3, 2017. Available from: http://www.nccn.org/professionals/physician_gls/pdf/esophageal.pdf. Accessed 10 January 2017.
Association of Comprehensive Cancer Centers Oesophageal carcinoma. Comprehensive Cancer Center, The Netherlands 2010. Available from: http://www.oncoline.nl/oesofaguscarcinoom. Accessed 10 January 2017.
Lordick F, Mariette C, Haustermans K, Obermannová R, Arnold D. ESMO guidelines committee. Oesophageal cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27:v50–7.
Monjazeb AM, Riedlinger G, Aklilu M, Geisinger KR, Mishra G, Isom S, et al. Outcomes of patients with esophageal cancer staged with [(1)(8)F]fluorodeoxyglucose positron emission tomography (FDG-PET): can postchemoradiotherapy FDG-PET predict the utility of resection? J Clin Oncol. Wake Forest University Health Sciences, Winston-Salem, NC 27103, USA. 2010;28:4714–21.
Smithers BM, Couper GC, Thomas JM, Wong D, Gotley DC, Martin I, et al. Positron emission tomography and pathological evidence of response to neoadjuvant therapy in adenocarcinoma of the esophagus. Dis Esophagus. 2008;21:151–8.
Findlay JM, Gillies RS, Franklin JM, Teoh EJ, Jones GE, di Carlo S, et al. Restaging oesophageal cancer after neoadjuvant therapy with (18)F-FDG PET-CT: identifying interval metastases and predicting incurable disease at surgery. Eur Radiol. 2016;26:3519–33.
Collins GS, Reitsma JB, Altman DG, Moons KGM. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. Ann Intern Med. 2015;162:55.
Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig L, et al. STARD 2015: an updated list of essential items for reporting diagnostic accuracy studies. Radiology. 2015;277:826–32.
Rice TW, Blackstone EH, Rusch VW. 7th edition of the AJCC Cancer staging manual: esophagus and esophagogastric junction. Ann Surg Oncol. 2010;17:1721–4.
Ajani JA, Correa AM, Hofstetter WL, Rice DC, Blum MA, Suzuki A, et al. Clinical parameters model for predicting pathologic complete response following preoperative chemoradiation in patients with esophageal cancer. Ann Oncol. 2012;23:2638–42.
Sudo K, Wang X, Xiao L, Wadhwa R, Shiozaki H, Elimova E, et al. A nomogram to predict distant metastases after multimodality therapy for patients with localized esophageal cancer. J Natl Compr Cancer Netw. 2016;14:173–9.
Shapiro J, van Klaveren D, Lagarde SM, Toxopeus ELA, van der Gaast A, Hulshof MCCM, et al. Prediction of survival in patients with oesophageal or junctional cancer receiving neoadjuvant chemoradiotherapy and surgery. Br J Surg. 2016;103:1039–47.
Nafteux PR, Lerut TE, Villeneuve PJ, Dhaenens JM, De Hertogh G, Moons J, et al. Signet ring cells in esophageal and gastroesophageal junction carcinomas have a more aggressive biological behavior. Ann Surg. 2014;260:1023–9.
Patel VR, Hofstetter WL, Correa AM, Agarwal A, Rashid A, Bhutani MS, et al. Signet ring cells in esophageal adenocarcinoma predict poor response to preoperative chemoradiation. Ann Thorac Surg. 2014;98:1064–71.
Xi M, Liao Z, Deng W, Xu C, Komaki R, Blum M, et al. A prognostic scoring model for the utility of induction chemotherapy prior to neoadjuvant chemoradiotherapy in esophageal cancer. J Thorac Oncol. 2017;12:1001–10.
Hayashi Y, Xiao L, Suzuki A, Blum MA, Sabloff B, Taketa T, et al. A nomogram associated with high probability of malignant nodes in the surgical specimen after trimodality therapy of patients with oesophageal cancer. Eur J Cancer. 2012;48:3396–404.
Nomura M, Shitara K, Kodaira T, Kondoh C, Takahari D, Ura T, et al. Recursive partitioning analysis for new classification of patients with esophageal cancer treated by chemoradiotherapy. Int J Radiat Oncol Biol Phys. 2012;84:786–92.
Dhar DK, Tachibana M, Kinukawa N, Riruke M, Kohno H, Little AG, et al. The prognostic significance of lymph node size in patients with squamous esophageal cancer. Ann Surg Oncol. 2002;9:1010–6.
de Boer AGEM, van Lanschot JJB, van Sandick JW, Hulscher JBF, Stalmeier PFM, de Haes JCJM, et al. Quality of life after transhiatal compared with extended transthoracic resection for adenocarcinoma of the esophagus. J Clin Oncol. 2004;22:4202–8.
Gabriel E, Alnaji R, Du W, Attwood K, Kukar M, Hochwald S. Effectiveness of repeat 18F-Fluorodeoxyglucose positron emission tomography computerized tomography (PET-CT) scan in identifying interval metastases for patients with esophageal cancer. Ann Surg Oncol. 2017;24:1739–46.
Suzuki A, Xiao L, Hayashi Y, Macapinlac HA, Welsh J, Lin SH, et al. Prognostic significance of baseline positron emission tomography and importance of clinical complete response in patients with esophageal or gastroesophageal junction cancer treated with definitive chemoradiotherapy. Cancer. 2011;117:4823–33.
Zhu Z-J, Hu Y, Zhao Y-F, Chen X-Z, Chen L-Q, Chen Y-T. Early recurrence and death after esophagectomy in patients with esophageal squamous cell carcinoma. Ann Thorac Surg. 2011;91:1502–8.
Lordick F, Ott K, Krause B-J, Weber WA, Becker K, Stein HJ, et al. PET to assess early metabolic response and to guide treatment of adenocarcinoma of the oesophagogastric junction: the MUNICON phase II trial. Lancet Oncol. 2007;8:797–805.
Cheedella NKS, Suzuki A, Xiao L, Hofstetter WL, Maru DM, Taketa T, et al. Association between clinical complete response and pathological complete response after preoperative chemoradiation in patients with gastroesophageal cancer: analysis in a large cohort. Ann Oncol. 2013;24:1262–6.
Kwee RM. Prediction of tumor response to neoadjuvant therapy in patients with esophageal cancer with use of 18 F FDG PET: a systematic review. Radiology. 2010;254:707–17.
Chen Y, Pan X, Tong L, Shi Y, Chen T. Can 18F-fluorodeoxyglucose positron emission tomography predict responses to neoadjuvant therapy in oesophageal cancer patients? A meta-analysis. Nucl Med Commun. 2011;32:1005–10.
Levine EA, Farmer MR, Clark P, Mishra G, Ho C, Geisinger KR, et al. Predictive value of 18-fluoro-deoxy-glucose-positron emission tomography (18F-FDG-PET) in the identification of responders to chemoradiation therapy for the treatment of locally advanced esophageal cancer. Ann Surg. 2006;243:472–8.
Grant MJ, Didier RA, Stevens JS, Beyder DD, Hunter JG, Thomas CR, et al. Radiation-induced liver disease as a mimic of liver metastases at serial PET/CT during neoadjuvant chemoradiation of distal esophageal cancer. Abdom Imaging. 2014;39:963–8.
Voncken FEM, Aleman BMP, van Dieren JM, Grootscholten C, Lalezari F, van Sandick JW, et al. Radiation-induced liver injury mimicking liver metastases on FDG-PET-CT after chemoradiotherapy for esophageal cancer. Strahlentherapie und Onkol. 2018;2:156–63.
Iyer RB, Balachandran A, Bruzzi JF, Johnson V, Macapinlac HA, Munden RF. PET/CT and hepatic radiation injury in esophageal cancer patients. Cancer Imaging. 2007;7:189–94.
FDG-PET/CT Technical Committee. FDG-PET/CT as an Imaging Biomarker Measuring Response to Cancer Therapy Profile, Quantitative Imaging Biomarkers Alliance. Version 1.05. Publicly Reviewed Version. QIBA, December 11, 2013. Available from: RSNA.org/QIBA. Accessed 1 March 2018.
Iasonos A, Schrag D, Raj GV, Panageas KS. How to build and interpret a nomogram for cancer prognosis. J Clin Oncol. 2008;26:1364–70.
Funding
Lucas Goense received a travel grant from the Rene Vogels Foundation to perform this research. No other funding was received for conducting this study.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
Steven H. Lin received research funding from STCube Pharmaceuticals, Genetech, Peregrine Pharmaceuticals, Hitachi Chemical, and honorarium from AstraZeneca. All other authors have no conflict of interest to declare.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
This retrospective study was approved by our Institutional Review Board, and the need for written informed consent was waived.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Goense, L., Ruurda, J.P., Carter, B.W. et al. Prediction and diagnosis of interval metastasis after neoadjuvant chemoradiotherapy for oesophageal cancer using 18F-FDG PET/CT. Eur J Nucl Med Mol Imaging 45, 1742–1751 (2018). https://doi.org/10.1007/s00259-018-4011-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-018-4011-6