Abstract
Purpose
Dynamic 18F-FET PET/CT is a powerful tool for the diagnosis of gliomas.18F-FET PET time–activity curves (TAC) allow differentiation between histological low-grade gliomas (LGG) and high-grade gliomas (HGG). Molecular methods such as epigenetic profiling are of rising importance for glioma grading and subclassification. Here, we analysed dynamic 18F-FET PET data, and the histological and epigenetic features of 44 gliomas.
Methods
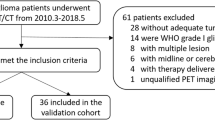
Dynamic 18F-FET PET was performed in 44 patients with newly diagnosed, untreated glioma: 10 WHO grade II glioma, 13 WHO grade III glioma and 21 glioblastoma (GBM). All patients underwent stereotactic biopsy or tumour resection after 18F-FET PET imaging. As well as histological analysis of tissue samples, DNA was subjected to epigenetic analysis using the Illumina 850 K methylation array. TACs, standardized uptake values corrected for background uptake in healthy tissue (SUVmax/BG), time to peak (TTP) and kinetic modelling parameters were correlated with histological diagnoses and with epigenetic signatures. Multivariate analyses were performed to evaluate the diagnostic accuracy of 18F-FET PET in relation to the tumour groups identified by histological and methylation-based analysis.
Results
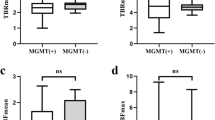
Epigenetic profiling led to substantial tumour reclassification, with six grade II/III gliomas reclassified as GBM. Overlap of HGG-typical TACs and LGG-typical TACs was dramatically reduced when tumours were clustered on the basis of their methylation profile. SUVmax/BG values of GBM were higher than those of LGGs following both histological diagnosis and methylation-based diagnosis. The differences in TTP between GBMs and grade II/III gliomas were greater following methylation-based diagnosis than following histological diagnosis. Kinetic modeling showed that relative K1 and fractal dimension (FD) values significantly differed in histology- and methylation-based GBM and grade II/III glioma between those diagnosed histologically and those diagnosed by methylation analysis. Multivariate analysis revealed slightly greater diagnostic accuracy with methylation-based diagnosis. IDH-mutant gliomas and GBM subgroups tended to differ in their 18F-FET PET kinetics.
Conclusion
The status of dynamic 18F-FET PET as a biologically and clinically relevant imaging modality is confirmed in the context of molecular glioma diagnosis.






Similar content being viewed by others
References
Langen KJ, Stoffels G, Filss C, Heinzel A, Stegmayr C, Lohmann P, et al. Imaging of amino acid transport in brain tumours: positron emission tomography with O-(2-[18F]fluoroethyl)-L-tyrosine (FET). Methods. 2017;130:124–34. https://doi.org/10.1016/j.ymeth.2017.05.019.
Albert NL, Weller M, Suchorska B, Galldiks N, Soffietti R, Kim MM, et al. Response Assessment in Neuro-Oncology working group and European Association for Neuro-Oncology recommendations for the clinical use of PET imaging in gliomas. Neuro Oncol. 2016;18(9):1199–208. https://doi.org/10.1093/neuonc/now058.
Galldiks N, Langen KJ, Pope WB. From the clinician’s point of view – what is the status quo of positron emission tomography in patients with brain tumors? Neuro Oncol. 2015;17(11):1434–44. https://doi.org/10.1093/neuonc/nov118.
Calcagni ML, Galli G, Giordano A, Taralli S, Anile C, Niesen A, et al. Dynamic O-(2-[18F]fluoroethyl)-L-tyrosine (F-18 FET) PET for glioma grading: assessment of individual probability of malignancy. Clin Nucl Med. 2011;36(10):841–7. https://doi.org/10.1097/RLU.0b013e3182291b40.
Popperl G, Kreth FW, Mehrkens JH, Herms J, Seelos K, Koch W, et al. FET PET for the evaluation of untreated gliomas: correlation of FET uptake and uptake kinetics with tumour grading. Eur J Nucl Med Mol Imaging. 2007;34(12):1933–42. https://doi.org/10.1007/s00259-007-0534-y.
Weckesser M, Langen KJ, Rickert CH, Kloska S, Straeter R, Hamacher K, et al. O-(2-[18F]fluorethyl)-L-tyrosine PET in the clinical evaluation of primary brain tumours. Eur J Nucl Med Mol Imaging. 2005;32(4):422–9. https://doi.org/10.1007/s00259-004-1705-8.
Dunet V, Pomoni A, Hottinger A, Nicod-Lalonde M, Prior JO. Performance of 18F-FET versus 18F-FDG-PET for the diagnosis and grading of brain tumors: systematic review and meta-analysis. Neuro Oncol. 2016;18(3):426–34. https://doi.org/10.1093/neuonc/nov148.
Pauleit D, Floeth F, Hamacher K, Riemenschneider MJ, Reifenberger G, Muller HW, et al. O-(2-[18F]fluoroethyl)-L-tyrosine PET combined with MRI improves the diagnostic assessment of cerebral gliomas. Brain. 2005;128(Pt 3):678–87. https://doi.org/10.1093/brain/awh399.
Rapp M, Heinzel A, Galldiks N, Stoffels G, Felsberg J, Ewelt C, et al. Diagnostic performance of 18F-FET PET in newly diagnosed cerebral lesions suggestive of glioma. J Nucl Med. 2013;54(2):229–35. https://doi.org/10.2967/jnumed.112.109603.
Albert NL, Winkelmann I, Suchorska B, Wenter V, Schmid-Tannwald C, Mille E, et al. Early static (18)F-FET-PET scans have a higher accuracy for glioma grading than the standard 20-40 min scans. Eur J Nucl Med Mol Imaging. 2016;43(6):1105–14. https://doi.org/10.1007/s00259-015-3276-2.
Jansen NL, Graute V, Armbruster L, Suchorska B, Lutz J, Eigenbrod S, et al. MRI-suspected low-grade glioma: is there a need to perform dynamic FET PET? Eur J Nucl Med Mol Imaging. 2012;39(6):1021–9. https://doi.org/10.1007/s00259-012-2109-9.
Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016;131(6):803–20. https://doi.org/10.1007/s00401-016-1545-1.
Soozangar N, Sadeghi MR, Jeddi F, Somi MH, Shirmohamadi M, Samadi N. Comparison of genome-wide analysis techniques to DNA methylation analysis in human cancer. J Cell Physiol. 2018;233(5):3968–81. https://doi.org/10.1002/jap.26176.
Reifenberger G, Wishing HG, Knobbed-Thomson CB, Weller M. Advances in the molecular genetics of gliomas – implications for classification and therapy. Nat Rev Clin Oncol. 2017;14(7):434–52. https://doi.org/10.1038/nrclinonc.2016.204.
Sturm D, Witt H, Hovestadt V, Khuong-Quang DA, Jones DT, Konermann C, et al. Hotspot mutations in H3F3A and IDH1 define distinct epigenetic and biological subgroups of glioblastoma. Cancer Cell. 2012;22(4):425–37. https://doi.org/10.1016/j.ccr.2012.08.024.
Wiestler B, Capper D, Sill M, Jones DT, Hovestadt V, Sturm D, et al. Integrated DNA methylation and copy-number profiling identify three clinically and biologically relevant groups of anaplastic glioma. Acta Neuropathol. 2014;128(4):561–71. https://doi.org/10.1007/s00401-014-1315-x.
Reuss DE, Sahm F, Schrimpf D, Wiestler B, Capper D, Koelsche C, et al. ATRX and IDH1-R132H immunohistochemistry with subsequent copy number analysis and IDH sequencing as a basis for an “integrated” diagnostic approach for adult astrocytoma, oligodendroglioma and glioblastoma. Acta Neuropathol. 2015;129(1):133–46. https://doi.org/10.1007/s00401-014-1370-3.
Kratochwil C, Combs SE, Leotta K, Afshar-Oromieh A, Rieken S, Debus J, et al. Intra-individual comparison of 18F-FET and 18F-DOPA in PET imaging of recurrent brain tumors. Neuro Oncol. 2014;16(3):434–40. https://doi.org/10.1093/neuonc/not199.
RCoreTeam. R: A language and environment for statistical computing. R Foundation for Statistical Computing. 2017.
Robin X, Turck N, Hainard A, Tiberti N, Lisacek F, Sanchez J-C, et al. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics. 2011;12:77.
Gusyatiner O, Hegi ME. Glioma epigenetics: from subclassification to novel treatment options. Semin Cancer Biol. 2017. https://doi.org/10.1016/j.semcancer.2017.11.010.
Jones DT, Jager N, Kool M, Zichner T, Hutter B, Sultan M, et al. Dissecting the genomic complexity underlying medulloblastoma. Nature. 2012;488(7409):100–5. https://doi.org/10.1038/nature11284.
Korshunov A, Chavez L, Sharma T, Ryzhova M, Schrimpf D, Stichel D, et al. Epithelioid glioblastomas stratify into established diagnostic subsets upon integrated molecular analysis. Brain Pathol. 2017. https://doi.org/10.1111/bpa.12566.
Sahm F, Schrimpf D, Stichel D, Jones DTW, Hielscher T, Schefzyk S, et al. DNA methylation-based classification and grading system for meningioma: a multicentre, retrospective analysis. Lancet Oncol. 2017;18(5):682–94. https://doi.org/10.1016/S1470-2045(17)30155-9.
Verger A, Stoffels G, Bauer EK, Lohmann P, Blau T, Fink GR, et al. Static and dynamic 18F-FET PET for the characterization of gliomas defined by IDH and 1p/19q status. Eur J Nucl Med Mol Imaging. 2018;45(3):443–51. https://doi.org/10.1007/s00259-017-3846-6.
Galldiks N, Stoffels G, Ruge MI, Rapp M, Sabel M, Reifenberger G, et al. Role of O-(2-18F-fluoroethyl)-L-tyrosine PET as a diagnostic tool for detection of malignant progression in patients with low-grade glioma. J Nucl Med. 2013;54(12):2046–54. https://doi.org/10.2967/jnumed.113.123836.
Jansen NL, Suchorska B, Wenter V, Eigenbrod S, Schmid-Tannwald C, Zwergal A, et al. Dynamic 18F-FET PET in newly diagnosed astrocytic low-grade glioma identifies high-risk patients. J Nucl Med. 2014;55(2):198–203. https://doi.org/10.2967/jnumed.113.122333.
Suchorska B, Giese A, Biczok A, Unterrainer M, Weller M, Drexler M, et al. Identification of time-to-peak on dynamic 18F-FET-PET as a prognostic marker specifically in IDH1/2 mutant diffuse astrocytoma. Neuro Oncol. 2018;20(2):279–88. https://doi.org/10.1093/neuonc/nox153.
Thiele F, Ehmer J, Piroth MD, Eble MJ, Coenen HH, Kaiser HJ, et al. The quantification of dynamic FET PET imaging and correlation with the clinical outcome in patients with glioblastoma. Phys Med Biol. 2009;54(18):5525–39. https://doi.org/10.1088/0031-9155/54/18/012.
Dimitrakopoulou-Strauss A, Seiz M, Tuettenberg J, Schmieder K, Eisenhut M, Haberkorn U, et al. Pharmacokinetic studies of 68Ga-labeled bombesin (68Ga-BZH3) and F-18 FDG PET in patients with recurrent gliomas and comparison to grading: preliminary results. Clin Nucl Med. 2011;36(2):101–8. https://doi.org/10.1097/RLU.0b013e318203bb24.
Dimitrakopoulou-Strauss A, Strauss LG, Mikolajczyk K, Burger C, Lehnert T, Bernd L, et al. On the fractal nature of dynamic positron emission tomography (PET) studies. World J Nucl Med. 2003;2(4):306–13.
Pyka T, Gempt J, Hiob D, Ringel F, Schlegel J, Bette S, et al. Textural analysis of pre-therapeutic [18F]-FET-PET and its correlation with tumor grade and patient survival in high-grade gliomas. Eur J Nucl Med Mol Imaging. 2016;43(1):133–41. https://doi.org/10.1007/s00259-015-3140-4.
Kunz M, Thon N, Eigenbrod S, Hartmann C, Egensperger R, Herms J, et al. Hot spots in dynamic (18)FET-PET delineate malignant tumor parts within suspected WHO grade II gliomas. Neuro Oncol. 2011;13(3):307–16. https://doi.org/10.1093/neuonc/noq196.
Jansen NL, Schwartz C, Graute V, Eigenbrod S, Lutz J, Egensperger R, et al. Prediction of oligodendroglial histology and LOH 1p/19q using dynamic [18F]FET-PET imaging in intracranial WHO grade II and III gliomas. Neuro Oncol. 2012;14(12):1473–80. https://doi.org/10.1093/neuonc/nos259.
Reuss DE, Mamatjan Y, Schrimpf D, Capper D, Hovestadt V, Kratz A, et al. IDH mutant diffuse and anaplastic astrocytomas have similar age at presentation and little difference in survival: a grading problem for WHO. Acta Neuropathol. 2015;129(6):867–73. https://doi.org/10.1007/s00401-015-1438-8.
Acknowledgements
We are gratefully to Karin Leotta (Division of Nuclear Medicine, University Hospital Heidelberg, Heidelberg, Germany) and Dr. Leyun Pan (German Cancer Research Center, Heidelberg, Germany) for their excellent support with the PMOD software for kinetic modelling.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts on interest
None.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the principles of the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Electronic supplementary material
ESM 1
(XLSX 13 kb)
Rights and permissions
About this article
Cite this article
Röhrich, M., Huang, K., Schrimpf, D. et al. Integrated analysis of dynamic FET PET/CT parameters, histology, and methylation profiling of 44 gliomas. Eur J Nucl Med Mol Imaging 45, 1573–1584 (2018). https://doi.org/10.1007/s00259-018-4009-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-018-4009-0