Abstract
Purpose
Treatment of late-stage prostate cancer by targeted radiotherapeutics such as 131I-MIP-1095 and 177Lu-PSMA-617 has shown encouraging early results. Lu-177 is preferred to I-131 in clinical settings, but targeted radioligand therapy (RLT) with 177Lu-PSMA-617 has not reached its full potential due to insufficient dose delivery to the tumor. We recently developed a dual-targeting radioiodinated ligand, RPS-027, that targets PSMA and uses albumin binding to enable good tumor uptake and significantly reduced kidney uptake in a preclinical model. Further development of this ligand is limited by the inability to independently modify PSMA and albumin binding and the requirement of I-131 for therapeutic application. We therefore sought to devise a new class of trifunctional ligands for RLT with (1) a high-affinity PSMA-binding domain, (2) an albumin-binding group (ABG), and (3) a chelator for radiometals such as 68Ga3+, 177Lu3+ and 225Ac3+.
Methods
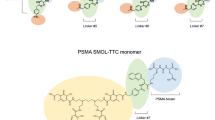
Ligands incorporating a triazolylphenylurea-containing PSMA-targeting group, an Nε-(2-(4-iodophenyl)acetyl)lysine ABG and the bifunctional chelator p-SCN-Bn-DOTA linked by a PEG-containing polymer containing 0,3,4,6,8 or 12 repeats were prepared. PSMA affinity was determined in LNCaP cells and uptake and tissue distribution was studied in mice bearing LNCaP tumor xenografts and compared to 177Lu-PSMA-617. Imaging studies were performed up to 24 h post-injection (p.i.) using 66Ga3+ and biodistribution studies at 4 h, 24 h and 96 h p.i. with 177Lu3+.
Results
PSMA affinity was high (IC50 = 1–10 nM) and inversely proportional to the linker length. Tumor uptake correlated with binding affinity and was significantly greater than for 177Lu-PSMA-617 over 96 h. The highest uptake was achieved with 177Lu-RPS-063 (30.0 ± 6.9 %ID/g; 4 h p.i.). Kidney uptake was generally high, with the exception of the lowest affinity ligand 177Lu-RPS-067. Each of the compounds showed slower blood clearance than 177Lu-PSMA-617, with clearance proportional to linker length.
Conclusions
The high tumor uptake achieved with these trifunctional ligands predicts larger (up to 4×) doses delivered to the tumor than can be achieved with 177Lu-PSMA-617. Although PSMA-mediated kidney uptake was also observed, the exceptional area under the curve (AUC) in the tumor warrants further investigation of these novel ligands as candidates for RLT.




Similar content being viewed by others
References
Nussbaum N, George DJ, Abernethy AP, Dolan CM, Oestreicher N, Flanders S, et al. Patient experience in the treatment of metastatic castration-resistant prostate cancer: state of the science. Prostate Cancer Prostatic Dis. 2016;19:111–21.
Zechmann CM, Afshar-Oromieh A, Armor T, Stubbs JB, Mier W, Hadaschik B, et al. Radiation dosimetry and first therapy results with a 124I/131I-labeled small molecule (MIP-1095) targeting PSMA for prostate cancer therapy. Eur J Nucl Med Mol Imaging. 2014;41:1280–92.
Afshar-Oromieh A, Haberkorn U, Zechmann C, Armor T, Mier W, Spohn F, et al. Repeated PSMA-targeting radioligand therapy of metastatic prostate cancer with 131I-MIP-1095. Eur J Nucl Med Mol Imaging. 2017;44:950–9.
Kratochwil C, Giesel FL, Stefanova M, Benešová M, Bronzel M, Afshar-Oromieh A, et al. PSMA-targeted radionuclide therapy of metastatic castration-resistant prostate cancer with 177Lu-labeled PSMA-617. J Nucl Med. 2016;57:1170–6.
Ahmadzadehfar H, Eppard E, Kürpig S, Fimmers R, Yordanova A, Schlenkhoff CD, et al. Therapeutic response and side effects of repeated radioligand therapy with 177Lu-PSMA-DKFZ-617 of castrate-resistant metastatic prostate cancer. Oncotarget. 2016;7:12477–88.
Fendler WP, Reinhardt S, Ilhan H, Delker A, Böning G, Gildehaus FJ, et al. Preliminary experience with dosimetry, response and patient reported outcome after 177Lu-PSMA-617 therapy for metastatic castration-resistant prostate cancer. Oncotarget. 2017;8:3581–90.
Rahbar K, Ahmadzadehfar H, Kratochwil C, Haberkorn U, Schäfers M, Essler M, et al. German multicenter study investigating 177Lu-PSMA-617 radioligand therapy in advanced prostate cancer patients. J Nucl Med. 2017;58:85–90.
Rahbar K, Schmidt M, Heinzel A, Eppard E, Bode A, Yordanova A, et al. Response and tolerability of a single dose of 177Lu-PSMA-617 in patients with metastatic castration-resistant prostate cancer: a multicenter retrospective analysis. J Nucl Med. 2016;57:1334–8.
Baum RP, Kulkarni HR, Schuchardt C, Singh A, Wirtz M, Wiessalla S, et al. Lutetium-177 PSMA radioligand therapy of metastatic castration-resistant prostate cancer: safety and efficacy. J Nucl Med. 2016;57:1006–13.
Heck MM, Retz M, D’Alessandria C, Rauschner I, Scheidhauer K, Maurer T, et al. Systemic radioligand therapy with 177Lu labeled prostate specific membrane antigen ligand for imaging and therapy in patients with metastatic castration resistant prostate cancer. J Urol. 2016;196:382–91.
Yordanova A, Becker A, Eppard E, Kürpig S, Fisang C, Feldmann G, et al. The impact of repeated cycles of radioligand therapy using [177Lu]Lu-PSMA-617 on renal function in patients with hormone refractory metastatic prostate cancer. Eur J Nucl Med Mol Imaging. 2017;44:1473–9.
Bräuer A, Grubert LS, Roll W, Schrader AJ, Schäfers M, Bögemann M, et al. 177Lu-PSMA-617 radioligand therapy and outcome in patients with metastasized castration-resistant prostate cancer. Eur J Nucl Med Mol Imaging. 2017;44:1663–70.
Rathke H, Giesel FL, Fleschig P, Kopka K, Mier W, Hohenfellner M, et al. Repeated Lu-177-PSMA-617 radioligand therapy using treatment activities up to 9.3 GBq. J Nucl Med. 2017; https://doi.org/10.2967/jnumed.117.194209.
Grimes J, Celler A. Comparison of internal dose estimates obtained using organ-level, voxel S value, and Monte Carlo techniques. Med Phys. 2014;41:092501.
Denis-Baceler AM, Chittenden SJ, Murray I, Divoli A, VR MC, Dearnaley DP, et al. A radiobiological model of metastatic burden reduction for molecular radiotherapy: application to patients with bone metastases. Phys Med Biol. 2017;62:2859–70.
Delker A, Fendler WP, Kratochwil C, Brunegraf A, Gosewisch A, Gildehaus FJ, et al. Dosimetry for 177Lu-DKFZ-PSMA-617: a new radiopharmaceutical for the treatment of metastatic prostate cancer. Eur J Nucl Med Mol Imaging. 2016;43:42–51.
Loke KSH, Padhy AK, Ng DCE, Goh ASW, Divgi C. Dosimetric considerations in radioimmunotherapy and systemic radionuclide therapies: a review. World J Nucl Med. 2011;10:122–38.
Hillier SM, Maresca KP, Lu G, Merkin RD, Marquis JC, Zimmerman CN, et al. 99mTc-labeled small-molecule inhibitors of prostate-specific membrane antigen for molecular imaging of prostate cancer. J Nucl Med. 2013;54:1369–76.
Kelly JM, Amor-Coarasa A, Nikolopoulou A, Wüstemann T, Barelli P, Kim D, Williams C., Jr, Zheng X, Bi C, Hu B, Warren JD, Hage DS, DiMagno SG, Babich JW. Dual-target binding ligands with modulated pharmacokinetics for endoradiotherapy of prostate cancer. J Nucl Med 2017;58:1442–1449.
Kelly J, Amor-Coarasa A, Nikolopoulou A, Kim D, Williams C Jr, Ponnala S, et al. Synthesis and pre-clinical evaluation of a new class of high-affinity 18F-labeled PSMA ligands for detection of prostate cancer by PET imaging. Eur J Nucl Med Mol Imaging. 2017;44:647–61.
Amor-Coarasa A, Milera A, Carvajal D, Gulec S, McGoron AJ. Lyophilized Kit for the Preparation of the PET Perfusion Agent [68Ga]-MAA. Int J Mol Imaging 2014; Article ID 269365: https://doi.org/10.1155/2014/269365.
Benešová M, Schäfer M, Bauder-Wüst U, Afshar-Oromieh A, Kratochwil C, Mier W, et al. Preclinical evaluation of a tailor-made DOTA-conjugated PSMA inhibitor with optimized linker moiety for imaging and endoradiotherapy of prostate cancer. J Nucl Med. 2015;56:914–20.
Kumar A, Mastren T, Wang B, Hsieh J-T, Hao G, Sun X. Design of a small-molecule drug conjugate for prostate cancer targeted theranostics. Bioconjug Chem. 2016;27:1681–9.
Choy CJ, Ling X, Geruntho JJ, Beyer SK, Latoche JD, Langton-Webster B, et al. 177Lu-labeled phosphoramidate-based PSMA inhibitors: the effect of an albumin binder on biodistribution and therapeutic efficacy in prostate tumor-bearing mice. Theranostics. 2017;7:1928–39.
Bao K, Lee JH, Kang H, Park GK, El Fakhri G, Choi HS. PSMA-targeted contrast agents for intraoperative imaging of prostate cancer. Chem Commun. 2017;53:1611–4.
Tykvart J, Schimer J, Bařinková J, Pachl P, Poštová-Slavĕtínská L, Majer P, et al. Rational design of urea-based glutamate carboxypeptidase II (GCPII) inhibitors as versatile tools for specific drug targeting and delivery. Bioorg Med Chem. 2014;22:4099–108.
Varasteh Z, Rosenström U, Velikyan I, Mitran B, Altai M, Honarvar H, et al. The effect of mini-PEG-based spacer length on binding and pharmacokinetic properties of a 68Ga-labeled NOTA-conjugated antagonistic analog of bombesin. Molecules. 2014;19:10455–72.
Weineisen M, Schottelius M, Simecek J, Baum RP, Yildiz A, Beykan S, et al. 68Ga- and 177Lu-labeled PSMA I&T: optimization of a PSMA-targeted theranostic concept and first proof-of-concept human studies. J Nucl Med. 2015;56:1169–76.
Kiess AP, Minn I, Chen Y, Hobbs R, Sgouros G, Mease RC, et al. Auger radiopharmaceutical therapy targeting prostate-specific membrane antigen. J Nucl Med. 2015;56:1401–7.
Hillier SM, Maresca KP, Femia FJ, Marquis JC, Foss CA, Nguyen N, et al. Preclinical evaluation of novel glutamate-urea-lysine analogs that target prostate specific membrane antigen as molecular imaging pharmaceuticals for prostate cancer. Cancer Res. 2009;69:6932–40.
Müller C, Struthers H, Winiger C, Zhernosekov K, Schibli R. DOTA conjugate with an albumin-binding entity enables the first folic acid–targeted 177Lu radionuclide tumor therapy in mice. J Nucl Med. 2013;54:121–31.
Kularatne SA, Wang K, Santhapuram HR, Low PS. Prostate-specific membrane antigen targeted imaging and therapy of prostate cancer using a PSMA inhibitor as a homing ligand. Mol Pharm. 2009;6:780–9.
Bacich DJ, Pinto JT, Tong WP, Heston WD. Cloning, expression, genomic localization, and enzymatic activities of the mouse homolog of prostate-specific membrane antigen/NAALADase/folate hydrolase. Mamm Genome. 2001;12:117–23.
Schmittgen TD, Zakrajsek BA, Hill RE, Liu Q, Reeves JJ, Axford PD, et al. Expression pattern of mouse homolog of prostate-specific membrane antigen (FOLHI) in the transgenic adenocarcinoma of the mouse prostate model. Prostate. 2003;55:308–16.
Chatalic KLS, Heskamp S, Konijnenberg M, Molkenboer-Kuenen JDM, Franssen GM, Clahsen-van Groningen MC, et al. Towards personalized treatment of prostate cancer: PSMA I&T, a promising prostate-specific membrane antigen-targeted theranostic agent. Theranostics. 2016;6:849–61.
Kratochwil C, Giesel FL, Leotta K, Eder M, Hoppe-Tich T, Youssoufian H, et al. PMPA for nephroprotection in PSMA-targeted radiotherapy of prostate cancer. J Nucl Med. 2015;56:293–8.
Urien S, Morin D, Tillement JP. Effect of alpha-1-acid glycoprotein, albumin and palmitic acid on the brain and salivary gland extraction of warfarin in rats. J Pharm Exp Ther. 1989;248:781–5.
Terasaki T, Pardridge WM, Denson DD. Differential effect of plasma protein binding of bupivacaine on its in vivo transfer into the brain and salivary gland of rats. J Pharm Exp Ther. 1986;239:724–9.
Gaertner FC, Halabi K, Ahmadzadehfar H, Kürpig S, Eppard E, Kotsikopoulous C, et al. Uptake of PSMA-ligands in normal tissues is dependent on tumor load in patients with prostate cancer. Oncotarget. 2017;8:55094–103.
Acknowledgements
This work was supported by a Pilot Award from the Weill Cornell Medical College Clinical and Translational Science Center, funded by NIH/NCATS UL1TR00457. The authors wish to thank Dr. J. David Warren of the Milstein Chemistry Core Facility at Weill Cornell Medicine for providing equipment for compound purification and access to equipment for reaction analysis and compound characterization. They would also like to acknowledge Dr. Yiauchung “Howard” Shen and Calvin Lom of Memorial-Sloan-Kettering Cancer Center for assistance with the production of Ga-66.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
James Kelly, Alejandro Amor-Coarasa, Shashikanth Ponnala, and John W. Babich are co-inventors on the constructs described in this manuscript and hold equity in Noria Therapeutics, Inc. Anastasia Nikolopoulou, Clarence Williams, Jr., David Schlyer, Yize Zhao, and Dohyun Kim declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Electronic supplementary material
ESM 1
(PDF 902 kb)
Rights and permissions
About this article
Cite this article
Kelly, J., Amor-Coarasa, A., Ponnala, S. et al. Trifunctional PSMA-targeting constructs for prostate cancer with unprecedented localization to LNCaP tumors. Eur J Nucl Med Mol Imaging 45, 1841–1851 (2018). https://doi.org/10.1007/s00259-018-4004-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-018-4004-5