Abstract
Purpose
The aim of the present study was to assess the value of interim 18F-FDG PET/CT performed after the first two cycles of ipilimumab treatment in the prediction of the final clinical response to this type of immunotherapy.
Methods
The study group comprised 41 patients with unresectable metastatic melanoma scheduled for ipilimumab therapy. Whole-body 18F-FDG PET/CT was performed before the start of ipilimumab treatment (baseline PET/CT) and after the initial two cycles of ipilimumab treatment (interim PET/CT). Evaluation of patient response to treatment was based on the European Organization for Research and Treatment of Cancer (EORTC) 1999 criteria for PET as well as the recently proposed PET Response Evaluation Criteria for Immunotherapy (PERCIMT). The patients’ best clinical response, assessed at a median of 21.4 months (range 6.3–41.9 months) was used as reference.
Results
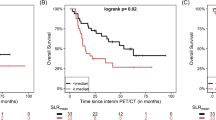
According to their best clinical response, the patients were divided into two groups: those showing clinical benefit (CB) including stable disease, partial response and complete response (31 patients), and those showing no clinical benefit (no-CB including progressive disease (10 patients). According to the EORTC criteria, interim PET/CT demonstrated progressive metabolic disease (PMD) in 20 patients, stable metabolic disease (SMD) in 11 patients, partial metabolic response (PMR) in 8 patients, and complete metabolic response (CMR) in 2 patients. According to the PERCIMT, interim PET/CT demonstrated PMD in 9 patients, SMD in 24 patients, PMR in 6 patients and CMR in 2 patients. On the basis of the interim PET, the patients were divided in a similar manner to the division according to clinical response into those showing metabolic benefit (MB) including SMD, PMR and CMR, and those showing no metabolic benefit (no-MB) including PMD. According to this dichotomization, the EORTC criteria showed a sensitivity (correctly predicting CB) of 64.5%, a specificity (correctly predicting no-CB) of 90.0%, a positive predictive value (PPV) of 95.2%, a negative predictive value (NPV) of 45.0% and an accuracy of 70.7% in predicting best clinical response. The PERCIMT showed a sensitivity of 93.6%, a specificity of 70.0%, a PPV of 90.6%, a NPV of 77.8% and an accuracy of 87.8%. The McNemar test showed that the PERCIMT had a significantly higher sensitivity than EORTC criteria (p = 0.004), while there was no significant difference in specificity (p = 0.5). The agreement between the two sets of criteria was poor (McNemar test p = 0.001, and accordingly kappa = 0.46).
Conclusion
The application of the recently proposed PERCIMT to interim 18F-FDG PET/CT provides a more sensitive predictor of final clinical response to immunotherapy than the application of the EORTC criteria in patients with metastatic melanoma.


Similar content being viewed by others
References
Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–23.
Gilardi L, Grana CM, Paganelli G. Evaluation of response to immunotherapy: new challenges and opportunities for PET imaging. Eur J Nucl Med Mol Imaging. 2014;41:2090–2.
Wolchok JD, Hoos A, O’Day S, Weber JS, Hamid O, Lebbé C, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15(23):7412–20.
Seymour L, Bogaerts J, Perrone A, Ford R, Schwartz LH, Mandrekar S, et al. RECIST working group. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 2017;18(3):e143–52.
Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: evolving considerations for PET response criteria in solid tumors. J Nucl Med. 2009;50(Suppl 1):122S–50S.
Holder WD Jr, White RL Jr, Zuger JH, Easton EJ Jr, Greene FL. Effectiveness of positron emission tomography for the detection of melanoma metastases. Ann Surg. 1998;227:764–9; discussion 769-771
Dietlein M, Krug B, Groth W, Smolarz K, Scheidhauer K, Psaras T, et al. Positron emission tomography using 18F-fluorodeoxyglucose in advanced stages of malignant melanoma: a comparison of ultrasonographic and radiological methods of diagnosis. Nucl Med Commun. 1999;20:255–61.
Eigtved A, Andersson AP, Dahlstrøm K, Rabøl A, Jensen M, Holm S, et al. Use of fluorine-18 fluorodeoxyglucose positron emission tomography in the detection of silent metastases from malignant melanoma. Eur J Nucl Med. 2000;27(1):70–5.
Mijnhout GS, Hoekstra OS, van Tulder MW, Teule GJ, Devillé WL. Systematic review of the diagnostic accuracy of 18F-fluorodeoxyglucose positron emission tomography in melanoma patients. Cancer. 2001;91:1530–42.
Swetter SM, Carroll LA, Johnson DL, Segall GM. Positron emission tomography is superior to computed tomography for metastatic detection in melanoma patients. Ann Surg Oncol. 2002;9:646–53.
Sachpekidis C, Larribere L, Pan L, Haberkorn U, Dimitrakopoulou-Strauss A, Hassel JC. Predictive value of early 18F-FDG PET/CT studies for treatment response evaluation to ipilimumab in metastatic melanoma: preliminary results of an ongoing study. Eur J Nucl Med Mol Imaging. 2015;42:386–96.
Cho SY, Lipson EJ, Im HJ, Rowe SP, Gonzalez EM, Blackford A, et al. Prediction of response to immune checkpoint inhibitor therapy using early time-point FDG-PET/CT imaging in patients with advanced melanoma. J Nucl Med. 2017;58:1421–8.
Seith F, Forschner A, Schmidt H, Pfannenberg C, Gückel B, Nikolaou K, et al. 18F-FDG-PET detects complete response to PD1-therapy in melanoma patients two weeks after therapy start. Eur J Nucl Med Mol Imaging. 2018;45:95–101.
Anwar H, Sachpekidis C, Winkler J, Kopp-Schneider A, Haberkorn U, Hassel JC, et al. Absolute number of new lesions on 18F-FDG PET/CT is more predictive of clinical response than SUV changes in metastatic melanoma patients receiving ipilimumab. Eur J Nucl Med Mol Imaging. 2018;45(3):376–83.
Young H, Baum R, Cremerius U, Herholz K, Hoekstra O, Lammertsma AA, et al. Measurement of clinical and subclinical tumour response using [18F]-fluorodeoxyglucose and positron emission tomography: review and 1999 EORTC recommendations. European Organization for Research and Treatment of Cancer (EORTC) PET Study Group. Eur J Cancer. 1999;35(13):1773–82.
Blodgett TM. Brain metastases. In: Blodgett TM, Ryan A, Almusa O, Papachristou M, Paidisetty S (eds). Specialty imaging. PET/CT oncologic imaging with correlative diagnostic CT. Salt Lake City: Amirsys; 2009. p. 14–22.
Hodi FS, Butler M, Obie DA, Seiden MV, Haluska FG, Kruse A, et al. Immunologic and clinical effects of antibody blockade of cytotoxic T lymphocyte-associated antigen 4 in previously vaccinated cancer patients. Proc Natl Acad Sci U S A. 2008;105:3005–10.
Dougan M, Dranoff G. Immune therapy for cancer. Annu Rev Immunol. 2009;27:83–117.
Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer. 1981;47:207–14.
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47.
Chiou VL, Burotto M. Pseudoprogression and immune-related response in solid tumors. J Clin Oncol. 2015;33(31):3541–3.
Hodi FS, Hwu WJ, Kefford R, Weber JS, Daud A, Hamid O, et al. Evaluation of immune-related response criteria and RECIST v1.1 in patients with advanced melanoma treated with pembrolizumab. J Clin Oncol. 2016;34:1510–7.
Koo PJ, Klingensmith WC, Lewis KD, Bagrosky BM, Gonzalez R. Anti-CTLA4 antibody therapy related complications on FDG PET/CT. Clin Nucl Med. 2014;39:e93–6.
DeVita VT Jr, Lawrence TS, Rosenberg SA. DeVita, Hellman, and Rosenberg’s cancer: principles & practice of oncology. 10th ed. Philadelphia: Wolters Kluwer; 2014.
Wachsmann JW, Ganti R, Peng F. Immune-mediated disease in Ipilimumab immunotherapy of melanoma with FDG PET-CT. Acad Radiol. 2017;24(1):111–5.
Downey SG, Klapper JA, Smith FO, Yang JC, Sherry RM, Royal RE, et al. Prognostic factors related to clinical response in patients with metastatic melanoma treated by CTL-associated antigen-4 blockade. Clin Cancer Res. 2007;13(22 Pt 1):6681–8.
Assi H, Wilson KS. Immune toxicities and long remission duration after ipilimumab therapy for metastatic melanoma: two illustrative cases. Curr Oncol. 2013;20:e165–16.
Attia P, Phan GQ, Maker AV, Robinson MR, Quezado MM, Yang JC, et al. Autoimmunity correlates with tumor regression in patients with metastatic melanoma treated with anti-cytotoxic T-lymphocyte antigen-4. J Clin Oncol. 2005;23(25):6043–53.
Nishino M, Giobbie-Hurder A, Gargano M, Suda M, Ramaiya NH, Hodi FS. Developing a common language for tumor response to immunotherapy: immune-related response criteria using unidimensional measurements. Clin Cancer Res. 2013;19(14):3936–43.
Kim JH. Comparison of the EORTC criteria and PERCIST in solid tumors: a pooled analysis and review. Oncotarget. 2016;7(36):58105–10.
Lin EC, Alavi A. PET and PET/CT: a clinical guide. New York: Thieme; 2009.
Funding
This study was supported in part by the German Cancer Aid under the project “Therapy monitoring of ipilimumab based on the quantification of F-18-FDG kinetics with 4D PET/CT (dPET-CT) in patients with melanoma (stage 4)”. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. No additional external funding was received for this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
Christos Sachpekidis reports travel grants from Janssen-Cilag outside the submitted work. Julia Winkler received speakers honoraria from MSD, and travel support from AMGEN, BMS, MSD, Philochem and Roche. Jessica C. Hassel received honoraria for talks and travel expenses from BMS, MSD, Roche, Novartis, Pfizer and is a member of an advisory board for MSD and Amgen. All other authors declare no conflicts of interest.
Ethical approval
The study was approved by the Ethics Committee of the University of Heidelberg (S-107/2012) and the Federal Agency for Radiation Protection (Bundesamt für Strahlenschutz).
Informed consent
Informed consent was obtained from all individual participants included in the study. This study did not include any studies with animals performed by any of the authors.
Additional information
Jessica C. Hassel and Antonia Dimitrakopoulou-Strauss share joint senior authorship.
Electronic supplementary material
ESM 1
(DOCX 98 kb)
Rights and permissions
About this article
Cite this article
Sachpekidis, C., Anwar, H., Winkler, J. et al. The role of interim 18F-FDG PET/CT in prediction of response to ipilimumab treatment in metastatic melanoma. Eur J Nucl Med Mol Imaging 45, 1289–1296 (2018). https://doi.org/10.1007/s00259-018-3972-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-018-3972-9