Abstract
Background
Validation of the prognostic value of the SIOPEN mIBG skeletal scoring system in two independent stage 4, mIBG avid, high-risk neuroblastoma populations.
Results
The semi-quantitative SIOPEN score evaluates skeletal meta-iodobenzylguanidine (mIBG) uptake on a 0–6 scale in 12 anatomical regions. Evaluable mIBG scans from 216 COG-A3973 and 341 SIOPEN/HR-NBL1 trial patients were reviewed pre- and post-induction chemotherapy. The prognostic value of skeletal scores for 5-year event free survival (5 yr.-EFS) was tested in the source and validation cohorts. At diagnosis, both cohorts showed a gradual non-linear increase in risk with cumulative scores. Several approaches were explored to test the relationship between score and EFS. Ultimately, a cutoff score of ≤3 was the most useful predictor across trials. A SIOPEN score ≤ 3 pre-induction was found in 15% SIOPEN patients and in 22% of COG patients and increased post-induction to 60% in SIOPEN patients and to 73% in COG patients. Baseline 5 yr.-EFS rates in the SIOPEN/HR-NBL1 cohort for scores ≤3 were 47% ± 7% versus 26% ± 3% for higher scores at diagnosis (p < 0.007) and 36% ± 4% versus 14% ± 4% (p < 0.001) for scores obtained post-induction. The COG-A3973 showed 5 yr.-EFS rates for scores ≤3 of 51% ± 7% versus 34% ± 4% for higher scores (p < 0.001) at diagnosis and 43% ± 5% versus 16% ± 6% (p = 0.004) for post-induction scores. Hazard ratios (HR) significantly favoured patients with scores ≤3 after adjustment for age and MYCN-amplification. Optimal outcomes were recorded in patients who achieved complete skeletal response.
Conclusions
Validation in two independent cohorts confirms the prognostic value of the SIOPEN skeletal score. In particular, patients with an absolute SIOPEN score > 3 after induction have very poor outcomes and should be considered for alternative therapeutic strategies.





Similar content being viewed by others
Abbreviations
- ASCT:
-
Autologous stem cell transplantation
- AUC:
-
Area under the curve
- CADO:
-
Cyclophosphamide, Adriamycin, Vincristine
- CBDCA:
-
Carboplatin
- CDDP:
-
Cisplatin
- CEM:
-
Carboplatin-Etoposide-Melphalan
- CI:
-
Confidence interval
- COG:
-
Children’s Oncology Group
- COJEC:
-
Rapid, platinum-containing induction schedule (CBDCA, CDDP, CYC, VCR, VP16)
- CR/PR:
-
Complete remission/partial remission
- CT:
-
Computed tomography
- CYC:
-
Cyclophosphamide
- DNA:
-
Deoxyribuncleic acid
- EFS:
-
Event free survival
- HDT:
-
High dose chemotherapy
- HR:
-
Hazard ratios
- HR-NBL:
-
High Risk Neuroblastoma
- INRG:
-
International Neuroblastoma Risk Group
- mIBG:
-
Metaiodobenzylguanidine
- MYCN:
-
Proto-oncogene
- PH:
-
Proportional hazard
- ROC:
-
Receiver operating characteristic
- SIOPEN:
-
Société International d’Oncologie Pédiatrique European Neuroblastoma
- SPECT:
-
Single-photon emission computed tomography
- TVD:
-
Topotecan, Vincristine, Doxorubicin
- VCR:
-
Vincristine
- VP16:
-
Etoposide
References
London WB, Castleberry RP, Matthay KK, Look AT, Seeger RC, Shimada H, et al. Evidence for an age cutoff greater than 365 days for neuroblastoma risk group stratification in the Children’s oncology group. J Clin Oncol. 2005;23:6459–65.
Moroz V, Machin D, Faldum A, Hero B, Iehara T, Mosseri V, et al. Changes over three decades in outcome and the prognostic influence of age-at-diagnosis in young patients with neuroblastoma: a report from the international neuroblastoma risk group project. Eur J Cancer. 2011;47:561–71.
Seeger RC, Brodeur GM, Sather H, Dalton A, Siegel SE, Wong KY, et al. Association of multiple copies of the N-myc oncogene with rapid progression of neuroblastomas. N Engl J Med. 1985;313:1111–6.
Shimada H, Stram DO, Chatten J, Joshi VV, Hachitanda Y, Brodeur GM, et al. Identification of subsets of neuroblastomas by combined histopathologic and N-myc analysis. J Natl Cancer Inst. 1995;87:1470–6.
Simon T, Hero B, Faldum A, Handgretinger R, Schrappe M, Klingebiel T, et al. Long term outcome of high-risk neuroblastoma patients after immunotherapy with antibody ch14.18 Or oral metronomic chemotherapy. BMC Cancer. 2011;11:21.
Valteau-Couanet D, Le Deley M-C, Bergeron C, Ducassou S, Michon J, Rubie H, et al. Long-term results of the combination of the N7 induction chemotherapy and the busulfan-melphalan high dose chemotherapy. Pediatr Blood Cancer. 2014;61:977–81.
Matthay KK, Reynolds CP, Seeger RC, Shimada H, Adkins ES, Haas-Kogan D, et al. Long-term results for children with high-risk neuroblastoma treated on a randomized trial of myeloablative therapy followed by 13-cis-retinoic acid: a children’s oncology group study. J Clin Oncol. 2009;27:1007–13.
Brodeur GM, Pritchard J, Berthold F, Carlsen NL, Castel V, Castelberry RP, et al. Revisions of the international criteria for neuroblastoma diagnosis, staging, and response to treatment. J Clin Oncol. 1993;11(8):1466–77.
Monclair T, Brodeur GM, Ambros PF, Brisse HJ, Cecchetto G, Holmes K, et al. The international neuroblastoma risk group (INRG) staging system: an INRG task force report. J Clin Oncol. 2009;27:298–303.
Matthay KK, Shulkin B, Ladenstein R, Michon J, Giammarile F, Lewington V, et al. Criteria for evaluation of disease extent by (123)I-metaiodobenzylguanidine scans in neuroblastoma: a report for the international neuroblastoma risk group (INRG) task force. Br J Cancer. 2010;102:1319–26.
Ladenstein R, Valteau-Couanet D, Brock P, Yaniv I, Castel V, Laureys G, et al. Randomized trial of prophylactic granulocyte colony-stimulating factor during rapid COJEC induction in pediatric patients with high-risk neuroblastoma: the European HR-NBL1/SIOPEN study. J Clin Oncol. 2010;28:3516–24.
Ladenstein R, Pötschger U, Pearson ADJ, Brock P, Luksch R, Castel V, et al. Busulfan and melphalan versus carboplatin, etoposide, and melphalan as high-dose chemotherapy for high-risk neuroblastoma (HR-NBL1/SIOPEN): an international, randomised, multi-arm, open-label, phase 3 trial. Lancet Oncol. 2017;18:500–14.
Lewington V, Lambert B, Poetschger U, Sever ZB, Giammarile F, McEwan AJB, et al. 123I-mIBG scintigraphy in neuroblastoma: development of a SIOPEN semi-quantitative reporting, method by an international panel. Eur J Nucl Med Mol Imaging. 2017;44:234–41.
Kreissman SG, Seeger RC, Matthay KK, London WB, Sposto R, Grupp SA, et al. Purged versus non-purged peripheral blood stem-cell transplantation for high-risk neuroblastoma (COG A3973): a randomised phase 3 trial. Lancet Oncol. 2013;14:999–1008.
Garaventa A, Luksch R, Biasotti S, Severi G, Pizzitola MR, Viscardi E, et al. A phase II study of topotecan with vincristine and doxorubicin in children with recurrent/refractory neuroblastoma. Cancer. 2003;98:2488–94.
Ladenstein R, Weixler S, Baykan B, Bleeke M, Kunert R, Katinger D, et al. Ch14.18 Antibody produced in CHO cells in relapsed or refractory stage 4 neuroblastoma patients: a SIOPEN phase 1 study. MAbs. 2013;5:801–9.
Yu AL, Gilman AL, Ozkaynak MF, London WB, Kreissman SG, Chen HX, et al. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N Engl J Med. 2010;363:1324–34.
Kaplan, E, Meier P. Nonparametric Estimation from Incomplete Observations. J Am Stat Assoc. 1958;53(282):457–81.
Marubini E, Valsecchi MG. Analysing survival data from clinical trials and observational studies. New York: Wiley; 2004.
Heinzl H, Kaider A. Gaining more flexibility in cox proportional hazards regression models with cubic spline functions. Comput Methods Prog Biomed. 1997;54:201–8.
Heagerty PJ, Zheng Y. Survival model predictive accuracy and ROC curves. Biometrics. 2005;61:92–105.
Heagerty PJ, Lumley T, Pepe MS. Time-dependent ROC curves for censored survival data and a diagnostic marker. Biometrics. 2000;56:337–44.
YOUDEN WJ. Index for rating diagnostic tests. Cancer. 1950:32–5.
Yanik GA, Parisi MT, Shulkin BL, Naranjo A, Kreissman SG, London WB, et al. Semiquantitative mIBG scoring as a prognostic indicator in patients with stage 4 neuroblastoma: a report from the Children’s oncology group. J Nucl Med. 2013;54:541–8.
Heagerty PJ. and packaging by PS-C. survivalROC: Time-dependent ROC curve estimation from censored survival data. 2013. p. R package version 1.0.3. 2013.
Decarolis B, Schneider C, Hero B, Simon T, Volland R, Roels F, et al. Iodine-123 metaiodobenzylguanidine scintigraphy scoring allows prediction of outcome in patients with stage 4 neuroblastoma: results of the cologne interscore comparison study. J Clin Oncol. 2013;31:944–51.
Yanik GA, Parisi MT, Naranjo A, Nadel H, Gelfand MJ, Park JR, et al. Validation of post-induction Curie scores in high risk neuroblastoma. A Children's Oncology Group (COG) and SIOPEN group report on SIOPEN/HR-NBL1. J Nucl Med. 2017. https://doi.org/10.2967/jnumed.117.195883
Hartmann O, Valteau-Couanet D, Vassal G, Lapierre V, Brugières L, Delgado R, et al. Prognostic factors in metastatic neuroblastoma in patients over 1 year of age treated with high-dose chemotherapy and stem cell transplantation: a multivariate analysis in 218 patients treated in a single institution. Bone Marrow Transplant. 1999;23:789–95.
Kushner BH, Kramer K, LaQuaglia MP, Modak S, Yataghene K, Cheung N-KV. Reduction from seven to five cycles of intensive induction chemotherapy in children with high-risk neuroblastoma. J Clin Oncol. 2004;22:4888–92.
Park JR, Kreissman SG, London WB, Naranjo A, Cohn SL, Hogarty MD, et al. A phase III randomized clinical trial (RCT) of tandem myeloablative autologous stem cell transplant (ASCT) using peripheral blood stem cell (PBSC) as consolidation therapy for high-risk neuroblastoma (HR-NB): A Children’s Oncology Group (COG) study. J Clin Oncol Am Soc Clin Oncol. 2016;34(suppl; abstr LBA3):LBA3–LBA3.
Ladenstein R, Philip T, Lasset C, Hartmann O, Garaventa A, Pinkerton R, et al. Multivariate analysis of risk factors in stage 4 neuroblastoma patients over the age of one year treated with megatherapy and stem-cell transplantation: a report from the European bone marrow transplantation solid tumor registry. J Clin Oncol. 1998;16:953–65.
Acknowledgements
We thank Marek Nykiel, Ingrid Pribill, PhD and Claudia Zeiner-Koglin, MSc at the Children’s Cancer Research Institute (CCRI) for their support.
Funding
This study was funded by the European Commission Community Research, Fifth Framework Program, Quality of Life and Management of living Resources: EC Grant No. QLRI-CT-2002-01768 (11. 2002–10. 2005) [https://www.siopen-r-net.org]; by the National Cancer Institute Paediatric and Adolescent Solid Tumour Steering Committee and received the following travel funding: Alex’s Lemonade Stand Foundation, Ben Towne Foundation, Children’s Neuroblastoma Cancer Foundation.
Author information
Authors and Affiliations
Contributions
Ruth Ladenstein and Bieke Lambert share first authorship.
Conception and design.
Ruth Ladenstein, Ulrike Pötschger, Valerie Lewington, Gregory Yanik, Katherine K. Matthay, Julie Park, Susan G. Kreissman, Ariane Boubaker.
Provision of study material or patients.
Ruth Ladenstein, Bieke Lambert, Valerie Lewington, Zvi Bar-Sever, Aurore Oudoux, Anna Śliwińska, Katerina Taborska, Lorenzo Biassoni, Gregory Yanik, Arlene Naranjo, Marguerite T. Parisi, Barry L. Shulkin, Helene Nadel, Michael J. Gelfand, Julie Park, Susan G. Kreissman, Dominique Valteau-Couanet, Ariane Boubaker.
Collection and assembly of data.
Ruth Ladenstein, Bieke Lambert, Maria-Rita Castellani, Zvi Bar-Sever, Aurore Oudoux, Anna Śliwińska, Katerina Taborska, Lorenzo Biassoni, Gregory Yanik, Arlene Naranjo, Marguerite T. Parisi, Barry L. Shulkin, Helene Nadel, Michael J. Gelfand, Julie Park, Susan G. Kreissman, Dominique Valteau-Couanet, Ariane Boubaker.
Data analysis and interpretation.
Ruth Ladenstein, Bieke Lambert, Ulrike Pötschger, Maria-Rita Castellani.
mIBG reviewers.
Bieke Lambert, Maria-Rita Castellani, Zvi Bar-Sever, Aurore Oudoux, Anna Śliwińska, Katerina Taborska, Lorenzo Biassoni, Ariane Boubaker.
Manuscript writing.
Ruth Ladenstein, Bieke Lambert, Ulrike Pötschger, Gregory Yanik, Ariane Boubaker.
Final approval of manuscript.
Ruth Ladenstein, Bieke Lambert, Ulrike Pötschger, Valerie Lewington, Maria-Rita Castellani, Zvi Bar-Sever, Aurore Oudoux, Anna Śliwińska, Katerina Taborska, Lorenzo Biassoni, Gregory Yanik, Arlene Naranjo, Marguerite T. Parisi, Barry L. Shulkin, Helene Nadel, Michael J. Gelfand, Katherine K. Matthay, Julie Park,
Susan G. Kreissman, Dominique Valteau-Couanet, Ariane Boubaker.
Corresponding author
Ethics declarations
Disclosure of potential conflict of interest
All authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in the analysis of the studies HR-NBL1/SIOPEN and COG-A3973 involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Part of data previously presented:
ASCO Annual Meeting 2014, Chicago, USA, May 30–June 3; J Clin Oncol 32:5 s, 2014 (suppl; abstr 10,029); EANM Annual Meeting Congress 2014, Oct 20–23, Gothenburg, Sweden
Ruth Ladenstein and Bieke Lambert share first authorship
Annex
Annex
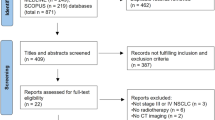
Annex 1: Consort Diagram

Annex 2
-
a)
Comparison of event-free survival (EFS) of patients on the SIOPEN trial and in the analysis mIBG review data set.

-
b)
Comparison of event-free survival (EFS) of patients on the COG A3973 trial and in the analysis mIBG review data set.

Rights and permissions
About this article
Cite this article
Ladenstein, R., Lambert, B., Pötschger, U. et al. Validation of the mIBG skeletal SIOPEN scoring method in two independent high-risk neuroblastoma populations: the SIOPEN/HR-NBL1 and COG-A3973 trials. Eur J Nucl Med Mol Imaging 45, 292–305 (2018). https://doi.org/10.1007/s00259-017-3829-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-017-3829-7