Abstract
Background
The objective of this study was to evaluate the potential of integrated 11C-MET PET/MR for response assessment of relapsed glioblastoma (GBM) receiving bevacizumab treatment.
Methods
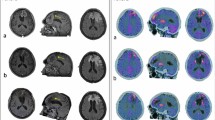
Eleven consecutive patients with relapsed GBM were enrolled for an integrated 11C-MET PET/MRI at baseline and at follow-up. Treatment response for MRI was evaluated according to Response Assessment in Neuro-oncology (RANO) criteria and integrated 11C-MET PET was assessed by the T/N ratio.
Results
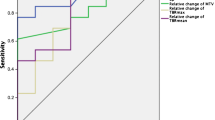
MRI showed no patient with complete response (CR), six of 11 patients with PR, four of 11 patients with SD, and one of 11 patients with progressive disease (PD). PET revealed metabolic response in five of the six patients with partial response (PR) and in two of the four patients with stable disease (SD), whereas metabolic non-response was detected in one of the six patients with PR, in two of the four patients with SD, and in the one patient with PD. Morphological imaging was predictive for PFS and OS when response was defined as CR, PR, SD, and non-response as PD. Metabolic imaging was predictive when using T/N ratio reduction of >25 as discriminator. Based on the morphologic and metabolic findings of this study a proposal for applying integrated PET/MRI for treatment response in relapsed GBM was developed, which was significantly predictive for PFS and OS (P = 0.010 respectively 0,029, log).
Conclusions
This study demonstrates the potential of integrated 11C-MET-PET/MRI for response assessment of GBM and the utility of combined assessment of morphologic and metabolic information with the proposal for assessing relapsed GBM.







Similar content being viewed by others
Abbreviations
- PET:
-
Positron emission tomography
- MRI:
-
Magnetic resonance imaging
- 11C-MET:
-
L-[methyl-11C] Methionine
- SUVmax:
-
Standardized uptake value of the tumor showing the maximum uptake
- MTV:
-
Metabolic tumor volume
- PFS:
-
Progression-free survival
- OS:
-
Overall survival
- c1:
-
Follow-up
References
Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–66. doi:10.1016/S1470-2045(09)70025-7.
Wong ET, Hess KR, Gleason MJ, Jaeckle KA, Kyritsis AP, Prados MD, et al. Outcomes and prognostic factors in recurrent glioma patients enrolled onto phase II clinical trials. J Clin Oncol Off J Am Soc Clin Oncol. 1999;17:2572–8.
Robles Irizarry L, Hambardzumyan D, Nakano I, Gladson CL, Ahluwalia MS. Therapeutic targeting of VEGF in the treatment of glioblastoma. Expert Opin Ther Targets. 2012;16:973–84. doi:10.1517/14728222.2012.711817.
Friedman HS, Prados MD, Wen PY, Mikkelsen T, Schiff D, Abrey LE, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol Off J Am Soc Clin Oncol. 2009;27:4733–40. doi:10.1200/JCO.2008.19.8721.
Wick W, Weller M, van den Bent M, Stupp R. Bevacizumab and recurrent malignant gliomas: a European perspective. J Clin Oncol Off J Am Soc Clin Oncol. 2010;28:e188–9. doi:10.1200/JCO.2009.26.9027. author reply e90–2.
Wick W, Brandes AA, Gorlia T, Bendszus M, Sahm F, Taal W, et al. EORTC 26101 phase III trial exploring the combination of bevacizumab and lomustine in patients with first progression of a glioblastoma. J Clin Oncol 2016;34:(suppl; abstr 2001).
Boss A, Bisdas S, Kolb A, Hofmann M, Ernemann U, Claussen CD, et al. Hybrid PET/MRI of intracranial masses: initial experiences and comparison to PET/CT. J Nucl Med Off Publ Soc Nucl Med. 2010;51:1198–205. doi:10.2967/jnumed.110.074773.
Wen PY, Macdonald DR, Reardon DA, Cloughesy TF, Sorensen AG, Galanis E, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol Off J Am Soc Clin Oncol. 2010;28:1963–72. doi:10.1200/JCO.2009.26.3541.
Galldiks N, Langen KJ, Pope WB. From the clinician’s point of view—what is the status quo of positron emission tomography in patients with brain tumors? Neuro-Oncology. 2015;17:1434–44. doi:10.1093/neuonc/nov118.
Kickingereder P, Wiestler B, Burth S, Wick A, Nowosielski M, Heiland S, et al. Relative cerebral blood volume is a potential predictive imaging biomarker of bevacizumab efficacy in recurrent glioblastoma. Neuro-Oncology. 2015;17:1139–47. doi:10.1093/neuonc/nov028.
Hu LS, Eschbacher JM, Dueck AC, Heiserman JE, Liu S, Karis JP, et al. Correlations between perfusion MR imaging cerebral blood volume, microvessel quantification, and clinical outcome using stereotactic analysis in recurrent high-grade glioma. AJNR Am J Neuroradiol. 2012;33:69–76. doi:10.3174/ajnr.A2743.
Nariai T, Tanaka Y, Wakimoto H, Aoyagi M, Tamaki M, Ishiwata K, et al. Usefulness of L-[methyl-11C] methionine-positron emission tomography as a biological monitoring tool in the treatment of glioma. J Neurosurg. 2005;103:498–507. doi:10.3171/jns.2005.103.3.0498.
Hutterer M, Nowosielski M, Putzer D, Waitz D, Tinkhauser G, Kostron H, et al. O-(2-18F-fluoroethyl)-L-tyrosine PET predicts failure of antiangiogenic treatment in patients with recurrent high-grade glioma. J Nucl Med Off Publ Soc Nucl Med. 2011;52:856–64. doi:10.2967/jnumed.110.086645.
Beppu T, Terasaki K, Sasaki T, Sato Y, Tomabechi M, Kato K, et al. MRI and 11C-methyl-L-methionine PET differentiate bevacizumab true responders after initiating therapy for recurrent glioblastoma. Clin Nucl Med. 2016;41:852–7. doi:10.1097/RLU.0000000000001377.
Dang L, White DW, Gross S, Bennett BD, Bittinger MA, Driggers EM, et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature. 2009;462:739–44. doi:10.1038/nature08617.
Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–96. doi:10.1056/NEJMoa043330.
Delso G, Furst S, Jakoby B, Ladebeck R, Ganter C, Nekolla SG, et al. Performance measurements of the Siemens mMR integrated whole-body PET/MR scanner. J Nucl Med Off Publ Soc Nucl Med. 2011;52:1914–22. doi:10.2967/jnumed.111.092726.
Quick HH. Integrated PET/MR. J Magn Reson Imaging JMRI. 2014;39:243–58. doi:10.1002/jmri.24523.
Young H, Baum R, Cremerius U, Herholz K, Hoekstra O, Lammertsma AA, et al. Measurement of clinical and subclinical tumour response using [18F]-fluorodeoxyglucose and positron emission tomography: review and 1999 EORTC recommendations. European Organization for Research and Treatment of Cancer (EORTC) PET Study Group. Eur J Cancer. 1999;35:1773–82.
Chen W, Delaloye S, Silverman DH, Geist C, Czernin J, Sayre J, et al. Predicting treatment response of malignant gliomas to bevacizumab and irinotecan by imaging proliferation with [18F] fluorothymidine positron emission tomography: a pilot study. J Clin Oncol Off J Am Soc Clin Oncol. 2007;25:4714–21. doi:10.1200/JCO.2006.10.5825.
Galldiks N, Ullrich R, Schroeter M, Fink GR, Jacobs AH, Kracht LW. Volumetry of [(11)C]-methionine PET uptake and MRI contrast enhancement in patients with recurrent glioblastoma multiforme. Eur J Nucl Med Mol Imaging. 2010;37:84–92. doi:10.1007/s00259-009-1219-5.
Yoo MY, Paeng JC, Cheon GJ, Lee DS, Chung JK, Kim EE, et al. Prognostic value of metabolic tumor volume on (11)C-methionine PET in predicting progression-free survival in high-grade glioma. Nucl Med Mol Imaging. 2015;49:291–7. doi:10.1007/s13139-015-0362-0.
Batra A, Tripathi RP, Singh AK. Perfusion magnetic resonance imaging and magnetic resonance spectroscopy of cerebral gliomas showing imperceptible contrast enhancement on conventional magnetic resonance imaging. Australas Radiol. 2004;48:324–32. doi:10.1111/j.0004-8461.2004.01315.x.
Herholz K, Holzer T, Bauer B, Schroder R, Voges J, Ernestus RI, et al. 11C-methionine PET for differential diagnosis of low-grade gliomas. Neurology. 1998;50:1316–22.
Galldiks N, Kracht LW, Burghaus L, Thomas A, Jacobs AH, Heiss WD, et al. Use of 11C-methionine PET to monitor the effects of temozolomide chemotherapy in malignant gliomas. Eur J Nucl Med Mol Imaging. 2006;33:516–24. doi:10.1007/s00259-005-0002-5.
Terakawa Y, Tsuyuguchi N, Iwai Y, Yamanaka K, Higashiyama S, Takami T, et al. Diagnostic accuracy of 11C-methionine PET for differentiation of recurrent brain tumors from radiation necrosis after radiotherapy. J Nucl Med Off Publ Soc Nucl Med. 2008;49:694–9. doi:10.2967/jnumed.107.048082.
Galldiks N, Rapp M, Stoffels G, Fink GR, Shah NJ, Coenen HH, et al. Response assessment of bevacizumab in patients with recurrent malignant glioma using [18F]fluoroethyl-L-tyrosine PET in comparison to MRI. Eur J Nucl Med Mol Imaging. 2013;40:22–33. doi:10.1007/s00259-012-2251-4.
Brandsma D, van den Bent MJ. Pseudoprogression and pseudoresponse in the treatment of gliomas. Curr Opin Neurol. 2009;22:633–8. doi:10.1097/WCO.0b013e328332363e.
Radbruch A, Lutz K, Wiestler B, Baumer P, Heiland S, Wick W, et al. Relevance of T2 signal changes in the assessment of progression of glioblastoma according to the Response Assessment in Neurooncology criteria. Neuro-Oncology. 2012;14:222–9. doi:10.1093/neuonc/nor200.
Radbruch A, Fladt J, Kickingereder P, Wiestler B, Nowosielski M, Baumer P, et al. Pseudoprogression in patients with glioblastoma: clinical relevance despite low incidence. Neuro-Oncology. 2015;17:151–9. doi:10.1093/neuonc/nou129.
Schmainda KM, Zhang Z, Prah M, Snyder BS, Gilbert MR, Sorensen AG, et al. Dynamic susceptibility contrast MRI measures of relative cerebral blood volume as a prognostic marker for overall survival in recurrent glioblastoma: results from the ACRIN 6677/RTOG 0625 multicenter trial. Neuro-Oncology. 2015;17:1148–56. doi:10.1093/neuonc/nou364.
Leu K, Pope WB, Cloughesy TF, Lai A, Nghiemphu PL, Chen W, et al. Imaging biomarkers for antiangiogenic therapy in malignant gliomas. CNS Oncol. 2013;2:33–47. doi:10.2217/cns.12.29.
Pope WB. Predictive imaging marker of bevacizumab efficacy: perfusion MRI. Neuro-Oncology. 2015;17:1046–7. doi:10.1093/neuonc/nov067.
Kickingereder P, Radbruch A, Burth S, Wick A, Heiland S, Schlemmer HP, et al. MR perfusion-derived hemodynamic parametric response mapping of Bevacizumab efficacy in recurrent glioblastoma. Radiology. 2016;279:542–52. doi:10.1148/radiol.2015151172.
Harris RJ, Cloughesy TF, Pope WB, Nghiemphu PL, Lai A, Zaw T, et al. 18F-FDOPA and 18F-FLT positron emission tomography parametric response maps predict response in recurrent malignant gliomas treated with bevacizumab. Neuro-Oncology. 2012;14:1079–89. doi:10.1093/neuonc/nos141.
Schwarzenberg J, Czernin J, Cloughesy TF, Ellingson BM, Pope WB, Geist C, et al. 3′-deoxy-3′-18F-fluorothymidine PET and MRI for early survival predictions in patients with recurrent malignant glioma treated with bevacizumab. J Nucl Med Off Publ Soc Nucl Med. 2012;53:29–36. doi:10.2967/jnumed.111.092387.
Kobayashi K, Hirata K, Yamaguchi S, Manabe O, Terasaka S, Kobayashi H, et al. Prognostic value of volume-based measurements on (11)C-methionine PET in glioma patients. Eur J Nucl Med Mol Imaging. 2015;42:1071–80. doi:10.1007/s00259-015-3046-1.
Preuss M, Werner P, Barthel H, Nestler U, Christiansen H, Hirsch FW, et al. Integrated PET/MRI for planning navigated biopsies in pediatric brain tumors. Childs Nerv Syst: ChNS Off J Int Soc Pediatr Neurosurg. 2014;30:1399–403. doi:10.1007/s00381-014-2412-9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
An IFORES grant to CD from the University Duisburg-Essen supported the research [http://www.uni-due.de/med/forschung/forschungsfoerderung/ifores.shtml]. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of interest
Nothing to disclosure.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Ethics Commission of the Medical Faculty of the University Duisburg-Essen and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study is registered at the Ethics Commission of the Medical Faculty of the University Duisburg-Essen with the number 11–4822-BO. Informed consent was obtained from all individual participants included in the study. This article does not contain any studies with animals performed by any of the authors.
Additional information
Joerg Hense and Marc Schlamann contributed equally to this work.
Rights and permissions
About this article
Cite this article
Deuschl, C., Moenninghoff, C., Goericke, S. et al. Response assessment of bevacizumab therapy in GBM with integrated 11C-MET-PET/MRI: a feasibility study. Eur J Nucl Med Mol Imaging 44, 1285–1295 (2017). https://doi.org/10.1007/s00259-017-3661-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-017-3661-0