Abstract
Purpose
The aim of this study was to assess the inter-rater variability of the visual interpretation of 11C-PiB PET images regarding the positivity/negativity of amyloid deposition that were obtained in a multicenter clinical research project, Japanese Alzheimer’s Disease Neuroimaging Initiative (J-ADNI). The results of visual interpretation were also compared with a semi-automatic quantitative analysis using mean cortical standardized uptake value ratio to the cerebellar cortex (mcSUVR).
Methods
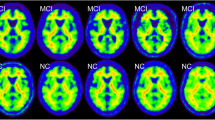
A total of 162 11C-PiB PET scans, including 45 mild Alzheimer’s disease, 60 mild cognitive impairment, and 57 normal cognitive control cases that had been acquired as J-ADNI baseline scans were analyzed. Based on visual interpretation by three independent raters followed by consensus read, each case was classified into positive, equivocal, and negative deposition (ternary criteria) and further dichotomized by merging the former two (binary criteria).
Results
Complete agreement of visual interpretation by the three raters was observed for 91.3% of the cases (Cohen κ = 0.88 on average) in ternary criteria and for 92.3% (κ = 0.89) in binary criteria. Cases that were interpreted as visually positive in the consensus read showed significantly higher mcSUVR than those visually negative (2.21 ± 0.37 vs. 1.27 ± 0.09, p < 0.001), and positive or negative decision by visual interpretation was dichotomized by a cut-off value of mcSUVR = 1.5. Significant positive/negative associations were observed between mcSUVR and the number of raters who evaluated as positive (ρ = 0.87, p < 0.0001) and negative (ρ = −0.85, p < 0.0001) interpretation. Cases of disagreement among raters showed generally low mcSUVR.
Conclusions
Inter-rater agreement was almost perfect in 11C-PiB PET scans. Positive or negative decision by visual interpretation was dichotomized by a cut-off value of mcSUVR = 1.5. As some cases of disagreement among raters tended to show low mcSUVR, referring to quantitative method may facilitate correct diagnosis when evaluating images of low amyloid deposition.




Similar content being viewed by others
References
McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack Jr CR, Kawas CH, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:263–9.
Karran E, Mercken M, De Strooper B. The amyloid cascade hypothesis for Alzheimer’s disease: an appraisal for the development of therapeutics. Nat Rev Drug Discov. 2011;10:698–712.
Jack Jr CR, Knopman DS, Jagust WJ, Shaw LM, Aisen PS, Weiner MW, et al. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol. 2010;9:119–28. doi:10.1016/S474-4422(09)70299-6.
Klunk W, Engler H, Nordberg A, Wang Y, Blomqvist G, Holt D, et al. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Ann Neurol. 2004;55:306–19.
Klunk WE, Koeppe RA, Price JC, Benzinger TL, Devous Sr MD, Jagust WJ, et al. The Centiloid Project: standardizing quantitative amyloid plaque estimation by PET. Alzheimers Dement. 2015;11:1–15.
Sabri O, Seibyl J, Rowe C, Barthel H. Beta-amyloid imaging with florbetaben. Clin Transl Imaging. 2015;3:13–26.
Curtis C, Gamez JE, Singh U, Sadowsky CH, Villena T, Sabbagh MN, et al. Phase 3 trial of flutemetamol labeled with radioactive fluorine 18 imaging and neuritic plaque density. JAMA Neurol. 2015;72:287–94.
Yang L, Rieves D, Ganley C. Brain amyloid imaging—FDA approval of florbetapir F18 injection. N Engl J Med. 2012;367:885–7. doi:10.1056/NEJMp1208061.
Hatashita S, Yamasaki H, Suzuki Y, Tanaka K, Wakebe D, Hayakawa H. [18F]Flutemetamol amyloid-beta PET imaging compared with [11C]PIB across the spectrum of Alzheimer’s disease. Eur J Nucl Med Mol Imaging. 2014;41:290–300.
Rabinovici G, Rosen H, Alkalay A, Kornak J, Furst A, Agarwal N, et al. Amyloid vs. FDG-PET in the differential diagnosis of AD and FTLD. Neurology. 2011;77:2034–42.
Ng S, Villemagne VL, Berlangieri S, Lee ST, Cherk M, Gong SJ, et al. Visual assessment versus quantitative assessment of 11C-PIB PET and 18F-FDG PET for detection of Alzheimer’s disease. J Nucl Med. 2007;48:547–52.
Rosario BL, Weissfeld LA, Laymon CM, Mathis CA, Klunk WE, Berginc MD, et al. Inter-rater reliability of manual and automated region-of-interest delineation for PiB PET. Neuroimage. 2011;55:933–41.
Akamatsu G, Ikari Y, Ohnishi A, Nishida H, Aita K, Sasaki M, et al. Automated PET-only quantification of amyloid deposition with adaptive template and empirically pre-defined ROI. Phys Med Biol. 2016;61:5768–80.
Suotunen T, Hirvonen J, Immonen-Raiha P, Aalto S, Lisinen I, Arponen E, et al. Visual assessment of [(11)C]PIB PET in patients with cognitive impairment. Eur J Nucl Med Mol Imaging. 2010;37:1141–7.
Tolboom N, van der Flier WM, Boverhoff J, Yaqub M, Wattjes MP, Raijmakers PG, et al. Molecular imaging in the diagnosis of Alzheimer’s disease: visual assessment of [11C]PIB and [18F]FDDNP PET images. J Neurol Neurosurg Psychiatry. 2010;81:882–4.
Seibyl J, Catafau AM, Barthel H, Ishii K, Rowe CC, Leverenz JB, et al. Impact of training method on the robustness of the visual assessment of 18F-Florbetaben PET scans: results from a phase-3 study. J Nucl Med. 2016;57:900–6.
Nayate AP, Dubroff JG, Schmitt JE, Nasrallah I, Kishore R, Mankoff D, et al. Use of standardized uptake value ratios decreases interreader variability of [18F] Florbetapir PET brain scan interpretation. AJNR Am J Neuroradiol. 2015;36:1237–44.
Yamane T, Ikari Y, Nishio T, Ishii K, Ishii K, Kato T, et al. Visual-statistical interpretation of (18)F-FDG-PET images for characteristic Alzheimer patterns in a multicenter study: inter-rater concordance and relationship to automated quantitative evaluation. AJNR Am J Neuroradiol. 2014;35:244–9.
Clark CM, Pontecorvo MJ, Beach TG, Bedell BJ, Coleman RE, Doraiswamy PM, et al. Cerebral PET with florbetapir compared with neuropathology at autopsy for detection of neuritic amyloid-β plaques: a prospective cohort study. Lancet Neurol. 2012;11:669–78.
Tolboom N, van der Flier WM, Yaqub M, Koene T, Boellaard R, Windhorst AD, et al. Differential association of [11C]PIB and [18F]FDDNP binding with cognitive impairment. Neurology. 2009;73:2079–85.
Grimmer T, Wutz C, Alexopoulos P, Drzezga A, Forster S, Forstl H, et al. Visual versus fully automated analyses of 18F-FDG and Amyloid PET for prediction of dementia due to Alzheimer disease in mild cognitive impairment. J Nucl Med. 2016;57:204–7.
Iwatsubo T. Japanese Alzheimer’s disease neuroimaging initiative: present status and future. Alzheimers Dement. 2010;6:297–9.
Ikari Y, Nishio T, Makishi Y, Miya Y, Ito K, Koeppe RA, et al. Head motion evaluation and correction for PET scans with 18F-FDG in the Japanese Alzheimer’s disease neuroimaging initiative (J-ADNI) multi-center study. Ann Nucl Med. 2012;26:535–44.
Japanese Alzheimer’s disease neuroimaging initiative (J-ADNI) (NBDC Research ID: hum0043.v1). National Bioscience Database Center website. http://humandbs.biosciencedbc.jp/en/hum0043-v1.
McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan E. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–44.
Braak H, Braak E. Frequency of stages of Alzheimer-related lesions in different age categories. Neurobiol Aging. 1997;18:351–7.
Delacourte A, David JP, Sergeant N, Buee L, Wattez A, Vermersch P, et al. The biochemical pathway of neurofibrillary degeneration in aging and Alzheimer’s disease. Neurology. 1999;52:1158–65.
Thurfjell L, Lilja J, Lundqvist R, Buckley C, Smith A, Vandenberghe R, et al. Automated quantification of 18F-flutemetamol PET activity for categorizing scans as negative or positive for brain amyloid: concordance with visual image reads. J Nucl Med. 2014;55:1623–8.
Schreiber S, Landau SM, Fero A, Schreiber F, Jagust WJ. Alzheimer’s disease neuroimaging I. Comparison of visual and quantitative Florbetapir F 18 positron emission tomography analysis in predicting mild cognitive impairment outcomes. JAMA Neurol. 2015;72:1183–90.
Chen ML. Ethnic or racial differences revisited: impact of dosage regimen and dosage form on pharmacokinetics and pharmacodynamics. Clin Pharmacokinet. 2006;45:957–64.
Senda M, Yamamoto Y, Sasaki M, Yamane T, Brooks DJ, Farrar G, et al. An exploratory efficacy study of the amyloid imaging agent [(18)F]flutemetamol in Japanese Subjects. Ann Nucl Med. 2015;29:391–9.
Senda M, Sasaki M, Yamane T, Shimizu K, Patt M, Barthel H, et al. Ethnic comparison of pharmacokinetics of (18)F-florbetaben, a PET tracer for beta-amyloid imaging, in healthy Caucasian and Japanese subjects. Eur J Nucl Med Mol Imaging. 2015;42:89–96.
Acknowledgments
The authors thank the J-ADNI Imaging Pharmaceutical Industry Scientific Advisory Board and other organizations for their support of this work.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Funding
This work is a part of the Translational Research Promotion Project/Research Project for the Development of a Systematic Method for the Assessment of Alzheimer’s Disease, sponsored by the New Energy and Industrial Technology Development Organization of Japan. The Japanese Alzheimer’s Disease Neuroimaging Initiative is also supported by a Grant-in-Aid for Comprehensive Research on Dementia from the Japanese Ministry of Health, Labour and Welfare, as well as by the grants from J-ADNI Pharmaceutical Industry Scientific Advisory Board companies.
Conflict of interest
TN has been an employee of Eli Lilly Japan since February 2016. However, all of his effort in the present study had been done when he was a member of Institute of Biomedical Research and Innovation. K. Ito has received research grants from Nihon Medi-Physics Co., Ltd. and a speaker honorarium from Eli Lilly Japan. MS is a principal investigator of clinical trials sponsored by AVID/Eli Lilly and GE Healthcare. MS is also an advisor for AVID/Eli Lilly, GE Healthcare and Piramal Imaging for which fee was paid to the institution.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the each institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Written informed consent was obtained from every subject prior to participation and also from caregiver of the subject categorized as AD or MCI.
Additional information
Research group of the Japanese Alzheimer’s Disease Neuroimaging Initiative (J-ADNI) comprised investigators from 38 different facilities. The investigators contributed to the design and implementation of J-ADNI and/or provided data but did not participate in the analyses of this report.
Rights and permissions
About this article
Cite this article
Yamane, T., Ishii, K., Sakata, M. et al. Inter-rater variability of visual interpretation and comparison with quantitative evaluation of 11C-PiB PET amyloid images of the Japanese Alzheimer’s Disease Neuroimaging Initiative (J-ADNI) multicenter study. Eur J Nucl Med Mol Imaging 44, 850–857 (2017). https://doi.org/10.1007/s00259-016-3591-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-016-3591-2