Abstract
Background
Z-endoxifen is the most potent of the metabolites of tamoxifen, and has the potential to be more effective than tamoxifen because it bypasses potential drug resistance mechanisms attributable to patient variability in the expression of the hepatic microsomal enzyme CYP2D6. 18F-FES is a positron emission tomography (PET) imaging agent which selectively binds to estrogen receptor alpha (ER-α) and has been used for non-invasive in vivo assessment of ER activity in tumors. This study utilizes 18F-FES PET imaging as a pharmacodynamic biomarker in patients with ER+ tumors treated with Z-endoxifen.
Methods
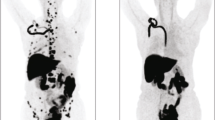
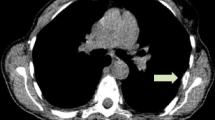
Fifteen patients were recruited from a parent therapeutic trial of Z-endoxifen and underwent imaging with 18F-FES PET at baseline. Eight had positive lesions on the baseline scan and underwent follow-up imaging with 18F-FES 1–5 days post administration of Z-endoxifen.
Results
Statistically significant changes (p = 0.0078) in standard uptake value (SUV)-Max were observed between the baseline and follow-up scans as early as 1 day post drug administration.
Conclusion
F-FES PET imaging could serve as a pharmacodynamic biomarker for patients treated with ER-directed therapy.





Similar content being viewed by others
References
Heldring N, Pike A, Andersson S, Matthews J, Cheng G, Hartman J, et al. Estrogen receptors: how do they signal and what are their targets. Physiol Rev. 2007;87(3):905–31. doi:10.1152/physrev.00026.2006.
Davies C, Godwin J, Gray R, Clarke M, Cutter D, Darby S, et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet. 2011;378(9793):771–84. doi:10.1016/S0140-6736(11)60993-8.
Ahmad A, Sheikh S, Kale P, Krishnappa M, Rane RC, Ahmad I. First-in-human study evaluating safety and pharmacokinetics of endoxifen, a potent estrogen-receptor antagonist for breast cancer. J Clin Oncol. 2010;28. doi:10.1200/jco.2010.28.15_suppl.3087.
Peterson LM, Mankoff DA, Lawton T, Yagle K, Schubert EK, Stekhova S, et al. Quantitative imaging of estrogen receptor expression in breast cancer with PET and 18F-fluoroestradiol. J Nucl Med. 2008;49(3):367–74. doi:10.2967/jnumed.107.047506.
Mortimer JE, Dehdashti F, Siegel BA, Trinkaus K, Katzenellenbogen JA, Welch MJ. Metabolic flare: indicator of hormone responsiveness in advanced breast cancer. J Clin Oncol. 2001;19(11):2797–803.
Mosselman S, Polman J, Dijkema R. ER beta: identification and characterization of a novel human estrogen receptor. FEBS Lett. 1996;392(1):49–53.
Honma N, Horii R, Iwase T, Saji S, Younes M, Takubo K, et al. Clinical importance of estrogen receptor-beta evaluation in breast cancer patients treated with adjuvant tamoxifen therapy. J Clin Oncol. 2008;26(22):3727–34. doi:10.1200/jco.2007.14.2968.
Younes M, Honma N. Estrogen receptor beta. Arch Pathol Lab Med. 2011;135(1):63–6. doi:10.1043/2010-0448-rar.1.
van Kruchten M, de Vries EG, Brown M, de Vries EF, Glaudemans AW, Dierckx RA, et al. PET imaging of oestrogen receptors in patients with breast cancer. Lancet Oncol. 2013;14(11):e465–75. doi:10.1016/s1470-2045(13)70292-4.
Fowler AM, Clark AS, Katzenellenbogen JA, Linden HM, Dehdashti F. Imaging diagnostic and therapeutic targets: steroid receptors in breast cancer. J Nucl Med. 2016;57 Suppl 1:75s–80. doi:10.2967/jnumed.115.157933.
Heidari P, Deng F, Esfahani SA, Leece AK, Shoup TM, Vasdev N, et al. Pharmacodynamic imaging guides dosing of a selective estrogen receptor degrader. Clin Cancer Res. 2015;21(6):1340–7. doi:10.1158/1078-0432.ccr-14-1178.
van Kruchten M, Hospers GA, Glaudemans AW, Hollema H, Arts HJ, Reyners AK. Positron emission tomography imaging of oestrogen receptor-expression in endometrial stromal sarcoma supports oestrogen receptor-targeted therapy: case report and review of the literature. Eur J Cancer (Oxford, England: 1990). 2013;49(18):3850–5. doi:10.1016/j.ejca.2013.08.005.
Linden HM, Kurland BF, Peterson LM, Schubert EK, Gralow JR, Specht JM, et al. Fluoroestradiol positron emission tomography reveals differences in pharmacodynamics of aromatase inhibitors, tamoxifen, and fulvestrant in patients with metastatic breast cancer. Clin Cancer Res. 2011;17(14):4799–805. doi:10.1158/1078-0432.ccr-10-3321.
Krause BJ, Souvatzoglou M, Herrmann K, Weber AW, Schuster T, Buck AK, et al. [11C]Choline as pharmacodynamic marker for therapy response assessment in a prostate cancer xenograft model. Eur J Nucl Med Mol Imaging. 2010;37(10):1861–8. doi:10.1007/s00259-010-1493-2.
Doran MG, Carnazza KE, Steckler JM, Spratt DE, Truillet C, Wongvipat J, et al. Applying (8)(9)Zr-transferrin to study the pharmacology of inhibitors to BET bromodomain containing proteins. Mol Pharm. 2016;13(2):683–8. doi:10.1021/acs.molpharmaceut.5b00882.
Yoo J, Dence CS, Sharp TL, Katzenellenbogen JA, Welch MJ. Synthesis of an estrogen receptor beta-selective radioligand: 5-[18F]fluoro-(2R,3S)-2,3-bis(4-hydroxyphenyl)pentanenitrile and comparison of in vivo distribution with 16alpha-[18F]fluoro-17beta-estradiol. J Med Chem. 2005;48(20):6366–78. doi:10.1021/jm050121f.
McGuire AH, Dehdashti F, Siegel BA, Lyss AP, Brodack JW, Mathias CJ, et al. Positron tomographic assessment of 16 alpha-[18F] fluoro-17 beta-estradiol uptake in metastatic breast carcinoma. J Nucl Med. 1991;32(8):1526–31.
Gemignani ML, Patil S, Seshan VE, Sampson M, Humm JL, Lewis JS, et al. Feasibility and predictability of perioperative PET and estrogen receptor ligand in patients with invasive breast cancer. J Nucl Med. 2013;54(10):1697–702. doi:10.2967/jnumed.112.113373.
Sundararajan L, Linden HM, Link JM, Krohn KA, Mankoff DA. 18F-Fluoroestradiol. Semin Nucl Med. 2007;37(6):470–6. doi:10.1053/j.semnuclmed.2007.08.003.
Linden HM, Stekhova SA, Link JM, Gralow JR, Livingston RB, Ellis GK, et al. Quantitative fluoroestradiol positron emission tomography imaging predicts response to endocrine treatment in breast cancer. J Clin Oncol. 2006;24(18):2793–9. doi:10.1200/jco.2005.04.3810.
Yoshida Y, Kiyono Y, Tsujikawa T, Kurokawa T, Okazawa H, Kotsuji F. Additional value of 16alpha-[18F]fluoro-17beta-oestradiol PET for differential diagnosis between uterine sarcoma and leiomyoma in patients with positive or equivocal findings on [18F]fluorodeoxyglucose PET. Eur J Nucl Med Mol Imaging. 2011;38(10):1824–31. doi:10.1007/s00259-011-1851-8.
Dixit M, Shi J, Wei L, Afari G, Bhattacharyya S. Synthesis of clinical-grade [(18)F]-fluoroestradiol as a surrogate PET biomarker for the evaluation of estrogen receptor-targeting therapeutic drug. Int J Mol Imaging. 2013;2013:278607. doi:10.1155/2013/278607.
Shi J, Afari G, Bhattacharyya S. Rapid synthesis of [18F]fluoroestradiol: remarkable advantage of microwaving over conventional heating. J label Compd Radiopharm. 2014;57(14):730–6. doi:10.1002/jlcr.3248.
Hu Z, Wang W, Gualtieri EE, Hsieh YL, Karp JS, Matej M, et al. An LOR-based fully-3D PET image reconstruction using a blob-basis function. 2007 I.E. Nuclear Science Symposium Conference Record. 2007;M26-228:4415–18. http://ieeexplore.ieee.org/document/4437091/. Accessed 19 May 2016.
van Kruchten M, de Vries EG, Glaudemans AW, van Lanschot MC, van Faassen M, Kema IP, et al. Measuring residual estrogen receptor availability during fulvestrant therapy in patients with metastatic breast cancer. Cancer Discov. 2015;5(1):72–81. doi:10.1158/2159-8290.cd-14-0697.
Peterson LM, Kurland BF, Schubert EK, Link JM, Gadi VK, Specht JM, et al. A phase 2 study of 16α-[(18)F]-fluoro-17β-estradiol positron emission tomography (FES-PET) as a marker of hormone sensitivity in metastatic breast cancer (MBC). Mol Imaging Biol: MIB. 2014;16(3):431–40. doi:10.1007/s11307-013-0699-7.
Yang Z, Sun Y, Zhang Y, Xue J, Wang M, Shi W, et al. Can fluorine-18 fluoroestradiol positron emission tomography-computed tomography demonstrate the heterogeneity of breast cancer in vivo? Clin Breast Cancer. 2013;13(5):359–63. doi:10.1016/j.clbc.2013.02.012.
van Kruchten M, de Vries EF, Arts HJ, Jager NM, Bongaerts AH, Glaudemans AW, et al. Assessment of estrogen receptor expression in epithelial ovarian cancer patients using 16alpha-18F-fluoro-17beta-estradiol PET/CT. J Nucl Med. 2015;56(1):50–5. doi:10.2967/jnumed.114.147579.
Zhao Z, Yoshida Y, Kurokawa T, Kiyono Y, Mori T, Okazawa H. 18F-FES and 18F-FDG PET for differential diagnosis and quantitative evaluation of mesenchymal uterine tumors: correlation with immunohistochemical analysis. J Nucl Med. 2013;54(4):499–506. doi:10.2967/jnumed.112.113472.
van Kruchten M, Glaudemans AW, de Vries EF, Schroder CP, de Vries EG, Hospers GA. Positron emission tomography of tumour [(18)F]fluoroestradiol uptake in patients with acquired hormone-resistant metastatic breast cancer prior to oestradiol therapy. Eur J Nucl Med Mol Imaging. 2015;42(11):1674–81. doi:10.1007/s00259-015-3107-5.
Zhang HY, Ke Q, Zhang Z, Zhang R, Fu J, Chen HJ, et al. Expression of beta-catenin and estrogen receptor in desmoid-type fibromatosis. Sichuan da Xue Xue Bao Yi Xue Ban = J Sichuan Univ Med Sci Ed. 2010;41(1):101–5.
Ishizuka M, Hatori M, Dohi O, Suzuki T, Miki Y, Tazawa C, et al. Expression profiles of sex steroid receptors in desmoid tumors. Tohoku J Exp Med. 2006;210(3):189–98.
Escobar C, Munker R, Thomas JO, Li BD, Burton GV. Update on desmoid tumors. Ann Oncol. 2012;23(3):562–9. doi:10.1093/annonc/mdr386.
Peterson LM, Kurland BF, Link JM, Schubert EK, Stekhova S, Linden HM, et al. Factors influencing the uptake of 18F-fluoroestradiol in patients with estrogen receptor positive breast cancer. Nucl Med Biol. 2011;38(7):969–78. doi:10.1016/j.nucmedbio.2011.03.002.
Kim BH, Kim SJ, Kim K, Kim H, Kim SJ, Kim WJ, et al. High metabolic tumor volume and total lesion glycolysis are associated with lateral lymph node metastasis in patients with incidentally detected thyroid carcinoma. Ann Nucl Med. 2015;29(8):721–9. doi:10.1007/s12149-015-0994-2.
Pak K, Cheon GJ, Nam HY, Kim SJ, Kang KW, Chung JK, et al. Prognostic value of metabolic tumor volume and total lesion glycolysis in head and neck cancer: a systematic review and meta-analysis. J Nucl Med. 2014;55(6):884–90. doi:10.2967/jnumed.113.133801.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This project was funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under contract no. HHSN261200800001E. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US government.
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. This study was approved by the NIH/NCI IRB.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Lin, F.I., Gonzalez, E.M., Kummar, S. et al. Utility of 18F-fluoroestradiol (18F-FES) PET/CT imaging as a pharmacodynamic marker in patients with refractory estrogen receptor-positive solid tumors receiving Z-endoxifen therapy. Eur J Nucl Med Mol Imaging 44, 500–508 (2017). https://doi.org/10.1007/s00259-016-3561-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-016-3561-8