Abstract
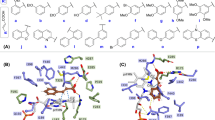
The enzyme ferulic acid decarboxylase (FADase) from Enterobacter sp. Px6-4 catalyzes the decarboxylation reaction of lignin monomers and phenolic compounds such as p-coumaric acid, caffeic acid, and ferulic acid into their corresponding 4-vinyl derivatives, that is, 4-vinylphenol, 4-vinylcatechol, and 4-vinylguaiacol, respectively. Among various ferulic acid decarboxylase enzymes, we chose the FADase from Enterobacter sp. Px6-4, whose crystal structure is known, and produced mutants to enhance its catalytic activity by random and site-directed mutagenesis. After three rounds of sequential mutations, FADase(F95L/D112N/V151I) showed approximately 34-fold higher catalytic activity than wild-type for the production of 4-vinylguaiacol from ferulic acid. Docking analyses suggested that the increased activity of FADase(F95L/D112N/V151I) could be due to formation of compact active site compared with that of the wild-type FADase. Considering the amount of phenolic compounds such as lignin monomers in the biomass components, successfully bioengineered FADase(F95L/D112N/V151I) from Enterobacter sp. Px6-4 could provide an ecofriendly biocatalytic tool for producing diverse styrene derivatives from biomass.


Similar content being viewed by others
References
Bradford M (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Cavin JF, Dartois V, Divies C (1998) Gene cloning, transcriptional analysis, purification, and characterization of phenolic acid decarboxylase from Bacillus subtilis. Appl Environ Microbiol 64:1466–1471
DOE (2002) Steam system opportunity assessment for the pulp and paper, chemical manufacturing, and petroleum refining industries—Main Report. US Department of Energy, Washington, DC
Gu W, Li X, Huang J, Duan Y, Meng Z, Zhang KQ, Yang J (2011) Cloning, sequencing, and overexpression in Escherichia coli of the Enterobacter sp. Px6-4 gene for ferulic acid decarboxylase. Appl Microbiol Biotechnol 89:1797–1805
Han D, Ryu J-Y, Lee H, Hur H-G (2013) Bacterial biotransformation of phenylpropanoid compounds for producing flavor and fragrance compounds. J Korean Soc Appl Biol Chem 56:125–133
Hanahan D (1985) Techniques for transformation of E. coli. DNA Cloning 1:109–135
Hatakeyama H, Hayashi E, Haraguchi T (1977) Biodegradation of poly(3-methoxy-4-hydroxy styrene). Polymer 18:759–763
Huang Z, Dostal L, Rosazza J (1993) Microbial transformations of ferulic acid by Saccharomyces cerevisiae and Pseudomonas fluorescens. Appl Environ Microbiol 59:2244–2250
Huang Z, Dostal L, Rosazza JP (1994) Purification and characterization of a ferulic acid decarboxylase from Pseudomonas fluorescens. J Bacteriol 176:5912–5918
Huang H, Chen L, Tokashiki M, Ozawa T, Taira T, Ito S (2012) An endogenous factor enhances ferulic acid decarboxylation catalyzed by phenolic acid decarboxylase from Candida guilliermondii. Appl Microbiol Biotechnol Express 2:1–10
Iwabuchi S, Nakahira T, Inohana A, Uchida H, Kojima K (1983) Polymeric catechol derivatives. IV. Polymerization behavior of 4-vinylcatechols and some properties of their polymeric derivatives. J Polym Sci Polym Chem Ed 21:1877–1884
Mathew S, Abraham T (2004) Ferulic acid: an antioxidant found naturally in plant cell walls and feruloyl esterases involved in its release and their applications. Crit Rev Biotechnol 24:59–83
Mathew S, Abraham TE (2006) Bioconversions of ferulic acid, an hydroxycinnamic acid. Crit Rev Microbiol 32:115–125
McKenna R, Nielsen D (2011) Styrene biosynthesis from glucose by engineered E. coli. Metab Eng 13:544–554
Mukai N, Masaki K, Fujii T, Kawamukai M, Iefuji H (2010) PAD1 and FDC1 are essential for the decarboxylation of phenylacrylic acids in Saccharomyces cerevisiae. J Biosci Bioeng 109:564–569
Narbad A, Gasson M (1998) Metabolism of ferulic acid via vanillin using a novel CoA-dependent pathway in a newly-isolated strain of Pseudomonas fluorescens. Microbiology 144:1397–1405
Pracht J, Boenigk J, Isenbeck-Schröter M, Keppler F, Schöler HF (2001) Abiotic Fe(III) induced mineralization of phenolic substances. Chemosphere 44:613–619
Priefert H, Rabenhorst J, Steinbüchel A (2001) Biotechnological production of vanillin. Appl Microbiol Biotechnol 56:296–314
Prim N, Pastor FIJ, Diaz P (2002) Zymographic detection of cinnamic acid decarboxylase activity. J Microbiol Methods 51:417–420
Qi W, Vannelli T, Breinig S, Ben-Bassat A, Gatenby A, Haynie S, Sariaslani FS (2007) Functional expression of prokaryotic and eukaryotic genes in Escherichia coli for conversion of glucose to hydroxystyrene. Metab Eng 9:268–276
Ranade SV, Richard RE, Helmus MN (2005) Styrenic block copolymers for biomaterial and drug delivery applications. Acta Biomater 1:137–144
Rodriguez H, Angulo I, De Las RB, Campillo N, Paez JA, Munoz R, Mancheno JM (2010) p-Coumaric acid decarboxylase from Lactobacillus plantarum: structural insights into the active site and decarboxylation catalytic mechanism. Proteins 78:1662–1676
Rosazza J, Huang Z, Dostal L, Volm T, Rousseau B (1995) Biocatalytic transformations of ferulic acid: an abundant aromatic natural product. J Ind Microbiol 15:457–471
Seo J, Kang S-I, Kim M, Han J, Hur H-G (2011) Flavonoids biotransformation by bacterial non-heme dioxygenases, biphenyl and naphthalene dioxygenase. Appl Microbiol Biotechnol 91:219–228
Stanier RY, Palleroni NJ, M. D (1966) The aerobic pseudomonads a taxonomic study. Microbiology 43:159-271
Verhoef S, Wierckx N, Westerhof R, de Winde J, Ruijssenaars H (2009) Bioproduction of p-hydroxystyrene from glucose by the solvent-tolerant bacterium Pseudomonas putida S12 in a two-phase water-decanol fermentation. Appl Environ Microbiol 75:931–936
Zago A, Degrassi G, Bruschi CV (1995) Cloning, sequencing, and expression in Escherichia coli of the Bacillus pumilus gene for ferulic acid decarboxylase. Appl Environ Microbiol 61:4484–4486
Acknowledgments
This work was supported by a grant from National Research Foundation grant (NRF:2010-0029224) of the Ministry of Education Science and Technology, Republic of Korea.
Conflict of interest
The authors declare that they have no competing interests.
Ethical statement
The authors followed principles of ethical and professional conduct in this research and in the preparation of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article has been retracted at the request of the authors.
The corresponding author, Hor-Gil Hur, reported to the Editor-in-Chief that the authors of this manuscript have raised concerns related to the accuracy of the data presented in this article. The authors determined that the enzyme activity data from the experiments performed by the authors are not consistent when tested repeatedly and the mutant does not show 34 fold higher catalytic activity compared to wild type which was the major claim in this manuscript. This is the major reason for the retraction of the manuscript. However, the authors did not find out the exact reason why it does not show reproducible data. It may be miscalculation or wrong interpretation of the data.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 195 kb)
About this article
Cite this article
Lee, H., Park, J., Jung, C. et al. RETRACTED ARTICLE: Enhancement of the catalytic activity of ferulic acid decarboxylase from Enterobacter sp. Px6-4 through random and site-directed mutagenesis. Appl Microbiol Biotechnol 99, 9473–9481 (2015). https://doi.org/10.1007/s00253-015-6717-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-015-6717-8