Abstract
Background
New pharmacovigilance legislation allows consumers to report adverse drug reactions (ADRs) directly to competent authorities in all European Union countries. Consumer reporting is available in Portugal since July 2012. In 2013, the National Pharmacovigilance System (SNF) had received 3461 spontaneous ADR reports, of which only 1.4 % (n = 50) were from consumers. Consumer reporting could be one opportunity to reduce underreporting.
Aim
The aim of this study was to describe the attitudes and knowledge of the general public regarding spontaneous reporting and the reasons and opinions that can influence consumers’ ADR underreporting.
Methods
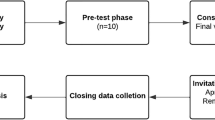
A descriptive-correlational study was performed looking for consumers’ attitudes and knowledge regarding spontaneous reporting. A 6-month survey was conducted from June to November 2013 in general adult consumers from a community pharmacy in Coimbra, Portugal, who used prescribed medicines or over-the-counter (OTC) drugs. Attitudes and opinions were surveyed by personal interview in a closed-answer questionnaire using a Likert scale. Questionnaires from healthcare professionals or incomplete ones were not considered. Data were analyzed using descriptive statistics, chi-square (χ 2) tests, and Spearman’s correlation coefficients.
Results
One thousand eighty-four questionnaires were collected (response rate of 81.1 %) and 948 completed were selected for analysis. Of the respondents, 44.1 % never heard about SNF. Younger people and those with a higher education were significantly more likely to be aware of SNF. Only one consumer had previously reported directly an ADR. Reporting ADRs indirectly through a healthcare professional (HCP) was preferred by 62.4 %. The main reasons for consumers reporting spontaneous ADR would be the severity of reactions (81.1 % agreed or strongly agreed) and worries about their situation (73.4 % agreed or strongly agreed). Only weak and moderate correlations were found between studied statements..
Conclusions
Consumers are more likely to do spontaneous report about severe reactions or if they are worried about the symptoms. Tailored and proactive information on ADR reporting and educational interventions on consumers could increase the number of reports from consumers in Portugal.
Similar content being viewed by others
References
Herdeiro MT, Figueiras A, Polónia J, Gestal-Otero J (2006) Influence of pharmacists’ attitudes on adverse drug reaction reporting. Drug Saf 29(4):331–340
Pal SN, Duncombe C, Falzon D, Olsson S (2013) WHO strategy for collecting safety data in public health programmes: complementing spontaneous reporting systems. Drug Saf 36(2):75–81
EC. Directive 2010/84/EU of the European Parliament and of the Council of 15 December 2010 amending, as regards pharmacovigilance. Directive 2001/83/EC on the Community code relating to medicinal products for human us [online]. Available from URL: http://eur-lex.europa.eu/LexUriServ/LexUriServ.douri=OJ:L:2010:348:0074:0099:EN:PDF [Accessed 2011 May 30], 2010.
Aagaard L, Nielsen LH, Hansen EH (2009) Consumer reporting of adverse drug reactions: a retrospective analysis of the Danish adverse drug reaction database from 2004 to 2006. Drug Saf 32(11):1067–1074
van Hunsel FPAM (2011) The contribution of direct patient reporting to pharmacovigilance
Avery AJ, Anderson C, Bond C, Fortnum H, Gifford A, Hannaford P, et al. (2011) Evaluation of patient reporting of adverse drug reactions to the UK 'Yellow Card Scheme': literature review, descriptive and qualitative analyses, and questionnaire surveys: Prepress Projects Limited
Blenkinsopp A, Wilkie P, Wang M, Routledge P (2006) Patient reporting of suspected adverse drug reactions: a review of published literature and international experience. Br J Clin Pharmacol 63(2):148–156
Hazell L, Cornelius V, Hannaford P, Shakir S, Avery AJ (2013) How do patients contribute to signal detection? Drug Saf 1-8
van Hunsel F, Talsma A, van Puijenbroek E, de Jong-van den Berg L, van Grootheest K. The proportion of patient reports of suspected ADRs to signal detection in the Netherlands: case–control study. Pharmacoepidemiol Drug Saf. 2011;20(3):286-291.
Marques J, Ribeiro-Vaz I, Pereira AC, Polónia J (2013) A survey of spontaneous reporting of adverse drug reactions in 10 years of activity in a pharmacovigilance centre in Portugal. Int J Pharm Pract
Anderson C, Krska J, Murphy E, Avery A (2011) The importance of direct patient reporting of suspected adverse drug reactions: a patient perspective. Br J Clin Pharmacol 72(5):806–822
Fortnum H, Lee A, Rupnik B, Avery A (2012) Survey to assess public awareness of patient reporting of adverse drug reactions in Great Britain. J Clin Pharm Ther 37(2):161–165
McLernon DJ, Bond CM, Lee AJ, Watson MC, Hannaford PC, Fortnum H, et al. (2011) Patient views and experiences of making adverse drug reaction reports to the Yellow Card Scheme in the UK. Pharmacoepidemiol Drug Saf 20(5):523–531
van Hunsel F, van der Welle C, Passier A, van Puijenbroek E, van Grootheest K (2010) Motives for reporting adverse drug reactions by patient-reporters in the Netherlands. Eur J Clin Pharmacol 66(11):1143–1150
Cohen J (1988) Statistical power analysis for the behavioral sciences
Inman W (1996) Attitudes to adverse drug reaction reporting. Br J Clin Pharmacol 41(5):434
INFARMED. Relatório anual 2013 - Notificações e Casos de RAM recebidos no SNF (Sistema Nacional de Farmacovigilância). 2014.
INFARMED. Relatório anual 2012 - Notificações e Casos de RAM recebidos no SNF (Sistema Nacional de Farmacovigilância). 2013.
Ekman E, Bäckström M (2009) Attitudes among hospital physicians to the reporting of adverse drug reactions in Sweden. Eur J Clin Pharmacol 65(1):43–46
Hasford J, Goettler M, Munter K-H, Müller-Oerlinghausen B (2002) Physicians’ knowledge and attitudes regarding the spontaneous reporting system for adverse drug reactions. J Clin Epidemiol 55(9):945–950
Belton K (1997) Attitude survey of adverse drug-reaction reporting by health care professionals across the European Union. Eur J Clin Pharmacol 52(6):423–427
Herdeiro MT, Figueiras A, Polónia J, Gestal-Otero JJ (2005) Physicians’ attitudes and adverse drug reaction reporting. Drug Saf 28(9):825–833
Herdeiro MT, Ribeiro-Vaz I, Ferreira M, Polónia J, Falcão A, Figueiras A (2012) Workshop- and telephone-based interventions to improve adverse drug reaction reporting. Drug Saf 35(8):655–665
Lopez-Gonzalez E, Herdeiro MT, Figueiras A (2009) Determinants of under-reporting of adverse drug reactions. Drug Saf 32(1):19–31
Ribeiro-Vaz I, Herdeiro MT, Polónia J, Figueiras A (2011) Strategies to increase the sensitivity of pharmacovigilance in Portugal. Rev Saude Publica 45(1):129–135
Van Grootheest AC (2003) Improving pharmacovigilance and the role of the pharmacist: Centre Lareb
Bäckström M, Mjörndal T, Dahlqvist R, Nordkvist-Olsson T (2000) Attitudes to reporting adverse drug reactions in northern Sweden. Eur J Clin Pharmacol 56(9-10):729–732
Biriell C, Edwards IR (1997) Reasons for reporting adverse drug reactions—some thoughts based on an international review. Pharmacoepidemiol Drug Saf 6(1):21–26
Golomb BA, McGraw JJ, Evans MA, Dimsdale JE (2007) Physician response to patient reports of adverse drug effects. Drug Saf 30(8):669–675
Frankenfeld C (2004) “Serious” and “severe” adverse drug reactions need defining. BMJ 329(7465):573
Rolfes L, Hunsel F, Wilkes S, Grootheest Kv, Puijenbroek Ev. Adverse drug reaction reports of patients and healthcare professionals—differences in reported information. Pharmacoepidemiol Drug Saf. 2014.
Cohen J (2013) Statistical power analysis for the behavioral sciences. Routledge Academic
Acknowledgments
No sources of funding were used to assist in the preparation of this manuscript.
Conflict of interest
The authors declare that they have no competing interests.
Author Contributions
All authors conceived and designed the study protocol.
Matos C. conducted the study, has collected and analyzed the data, and was the primary author of the manuscript.
Van Hunsel F. and Joaquim J. were the supervisors of the study and assisted with the manuscript preparation.
All authors contributed to the study design discussions and revision of the manuscript and gave the approval of final versions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Matos, C., van Hunsel, F. & Joaquim, J. Are consumers ready to take part in the Pharmacovigilance System?—a Portuguese preliminary study concerning ADR reporting. Eur J Clin Pharmacol 71, 883–890 (2015). https://doi.org/10.1007/s00228-015-1867-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-015-1867-2