Abstract
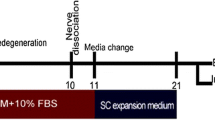
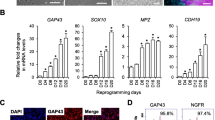
Schwann cells (SCs) are the supporting cells of the peripheral nervous system and originate from the neural crest. They play a unique role in the regeneration of injured peripheral nerves and have themselves a highly unstable phenotype as demonstrated by their unexpectedly broad differentiation potential. Thus, SCs can be considered as dormant, multipotent neural crest-derived progenitors or stem cells. Upon injury they de-differentiate via cellular reprogramming, re-enter the cell cycle and participate in the regeneration of the nerve. Here we describe a protocol for efficient generation of neurospheres from intact adult rat and murine sciatic nerve without the need of experimental in vivo pre-degeneration of the nerve prior to Schwann cell isolation. After isolation and removal of the connective tissue, the nerves are initially plated on poly-D-lysine coated cell culture plates followed by migration of the cells up to 80 % confluence and a subsequent switch to serum-free medium leading to formation of multipotent neurospheres. In this context, migration of SCs from the isolated nerve, followed by serum-free cultivation of isolated SCs as neurospheres mimics the injury and reprograms fully differentiated SCs into a multipotent, neural crest-derived stem cell phenotype. This protocol allows reproducible generation of multipotent Schwann cell-derived neurospheres from sciatic nerve through cellular reprogramming by culture, potentially marking a starting point for future detailed investigations of the de-differentiation process.





Similar content being viewed by others
References
Kaltschmidt, B., Kaltschmidt, C., & Widera, D. (2011). Adult craniofacial stem cells: sources and relation to the neural crest. Stem Cell Reviews. doi:10.1007/s12015-011-9340-9.
Schwann, T. (1839). Mikroskopische Untersuchungen über die Uebereinstimmung in der Struktur und dem Wachsthum der Thiere und Pflanzen. Berlin: Verlag der Sanderschen Buchhandlung.
Bunge, R. P. (1993). Expanding roles for the Schwann cell: ensheathment, myelination, trophism and regeneration. Current Opinion in Neurobiology, 3, 805–809.
Fawcett, J. W., & Keynes, R. J. (1990). Peripheral nerve regeneration. Annual Review of Neuroscience, 13, 43–60.
Mirsky, R., Jessen, K. R., Brennan, A., et al. (2002). Schwann cells as regulators of nerve development. Journal of Physiology, Paris, 96, 17–24.
Sherman, L., Stocker, K. M., Morrison, R., & Ciment, G. (1993). Basic fibroblast growth factor (bFGF) acts intracellularly to cause the transdifferentiation of avian neural crest-derived Schwann cell precursors into melanocytes. Development, 118, 1313–1326.
Dupin, E., Real, C., Glavieux-Pardanaud, C., Vaigot, P., & Le Douarin, N. M. (2003). Reversal of developmental restrictions in neural crest lineages: transition from Schwann cells to glial-melanocytic precursors in vitro. Proceedings of the National Academy of Sciences of the United States of America, 100, 5229–5233.
Real, C., Glavieux-Pardanaud, C., Vaigot, P., Le-Douarin, N., & Dupin, E. (2005). The instability of the neural crest phenotypes: Schwann cells can differentiate into myofibroblasts. The International Journal of Developmental Biology, 49, 151–159.
Binder, E., Rukavina, M., Hassani, H., et al. (2011). Peripheral nervous system progenitors can be reprogrammed to produce myelinating oligodendrocytes and repair brain lesions. The Journal of Neuroscience: The Official Journal of The Society for Neuroscience, 31, 6379–6391.
Widera, D., Heimann, P., Zander, C., et al. (2011). Schwann cells can be reprogrammed to multipotency by culture. Stem Cells and Development, 20, 2053–2064.
Morrissey, T. K., Kleitman, N., & Bunge, R. P. (1991). Isolation and functional characterization of Schwann cells derived from adult peripheral nerve. The Journal of Neuroscience: The Official Journal of The Society for Neuroscience, 11, 2433–2442.
Takagi, T., Ishii, K., Shibata, S., et al. (2011). Schwann-spheres derived from injured peripheral nerves in adult mice–their in vitro characterization and therapeutic potential. PloS One, 6, e21497.
Popovic, M., Sketelj, J., & Bresjanac, M. (1996). Changes of Schwann cell antigenic profile after peripheral nerve injury. Pflugers Archiv: European Journal of Physiology, 431, R287–R288.
Rutkowski, J. L., Tennekoon, G. I., & McGillicuddy, J. E. (1992). Selective culture of mitotically active human Schwann cells from adult sural nerves. Annals of Neurology, 31, 580–586.
Keilhoff, G., Fansa, H., Schneider, W., & Wolf, G. (1999). In vivo predegeneration of peripheral nerves: an effective technique to obtain activated Schwann cells for nerve conduits. Journal of Neuroscience Methods, 89, 17–24.
Verdu, E., Rodriguez, F. J., Gudino-Cabrera, G., Nieto-Sampedro, M., & Navarro, X. (2000). Expansion of adult Schwann cells from mouse predegenerated peripheral nerves. Journal of Neuroscience Methods, 99, 111–117.
de la Fuente, I., Alcalde, I., Gamboa, O. L., Garrosa, M., & Gayoso, M. J. (2012). A method for obtaining Schwann cell cultures from adult rabbit nerve based on “in vitro” pre-degeneration and neuregulin treatment. Histology and Histopathology, 27, 95–102.
Simm, A., Bertsch, G., Frank, H., Zimmermann, U., & Hoppe, J. (1997). Cell death of AKR-2B fibroblasts after serum removal: a process between apoptosis and necrosis. Journal of Cell Science, 110(Pt 7), 819–828.
Russel, S. A. (2001). Molecular cloning: a laboratory manual. 1.
Chen, Y., Stevens, B., Chang, J., Milbrandt, J., Barres, B. A., & Hell, J. W. (2008). NS21: re-defined and modified supplement B27 for neuronal cultures. Journal of Neuroscience Methods, 171, 239–247.
Acknowledgments
This study was supported by a FiF grant of the Bielefeld University to DW.
Conflicts of interest
The authors declare no potential conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Martin, I., Nguyen, T.D., Krell, V. et al. Generation of Schwann Cell-Derived Multipotent Neurospheres Isolated from Intact Sciatic Nerve. Stem Cell Rev and Rep 8, 1178–1187 (2012). https://doi.org/10.1007/s12015-012-9387-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-012-9387-2