Abstract
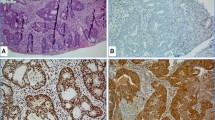
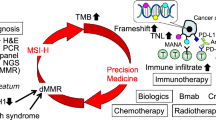
High-frequency microsatellite instability (MSI-H) accounts for roughly 15 % of all cases of colorectal cancer (CRC) and results from pathogenic mutations or epigenetic changes in mismatch repair (MMR) proteins, primarily MLH1, MSH2, MSH6, and PMS2. These alterations can be inherited, as in the case of Lynch syndrome, or can be acquired sporadically, including cases of epigenetic alteration along crucial regulatory sequences. Cancers that develop in the setting of MSI-H possess a unique clinicopathologic phenotype, with a high degree of mutation resulting in potential recognition by the immune system. These features have directed therapeutic investigation in recent years to involve consideration of immune-stimulating agents, which might exploit the inherent immunogenicity of these tumors.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA: Cancer J Clin. 2015;65(1):5–29.
Chan AT, Giovannucci EL. Primary prevention of colorectal cancer. Gastroenterology. 2010;138(6):2029–43. e10.
Leslie A et al. The colorectal adenoma–carcinoma sequence. Br J Surg. 2002;89(7):845–60.
Bettington M et al. The serrated pathway to colorectal carcinoma: current concepts and challenges. Histopathology. 2013;62(3):367–86.
Grady W. Genomic instability and colon cancer. Cancer Metastasis Rev. 2004;23(1–2):11–27.
Pino MS, Chung DC. The chromosomal instability pathway in colon cancer. Gastroenterology. 2010;138(6):2059–72.
Al-Sohaily S et al. Molecular pathways in colorectal cancer. J Gastroenterol Hepatol. 2012;27(9):1423–31.
Boland CR, Goel A. Microsatellite instability in colorectal cancer. Gastroenterology. 2010;138(6):2073–87. e3.
Esteller M. Epigenetics in cancer. N Engl J Med. 2008;358(11):1148–59.
Hughes LAE et al. The CpG island methylator phenotype in colorectal cancer: progress and problems. Biochimica et Biophysica Acta (BBA) - Rev Cancer. 2012;1825(1):77–85.
Sinicrope FA, Sargent DJ. Molecular pathways: microsatellite instability in colorectal cancer: prognostic, predictive, and therapeutic implications. Clin Cancer Res: Off J Am Assoc Cancer Res. 2012;18(6):1506–12.
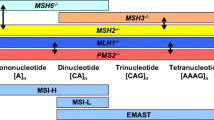
Geiersbach KB, Samowitz WS. Microsatellite instability and colorectal cancer. Archives Pathol Laboratory Med. 2011;135(10):1269–77.
Dietmaier W et al. Diagnostic microsatellite instability: definition and correlation with mismatch repair protein expression. Cancer Res. 1997;57(21):4749–56.
Tomlinson I et al. Does MSI-low exist? J Pathol. 2002;197(1):6–13.
Halford S et al. Low-level microsatellite instability occurs in most colorectal cancers and is a nonrandomly distributed quantitative trait. Cancer Res. 2002;62(1):53–7.
González-García I et al. Standardized approach for microsatellite instability detection in colorectal carcinomas. J Natl Cancer Inst. 2000;92(7):544–9.
Pawlik TM, Raut CP, Rodriguez-Bigas MA. Colorectal carcinogenesis: MSI-H versus MSI-L. Dis Markers. 2004;20(4–5):199–206.
Peltomaki P, Vasen HF. Mutations predisposing to hereditary nonpolyposis colorectal cancer: database and results of a collaborative study. The international collaborative group on hereditary nonpolyposis colorectal cancer. Gastroenterology. 1997;113(4):1146–58.
Bonadona V et al. CAncer risks associated with germline mutations in mlh1, msh2, and msh6 genes in lynch syndrome. JAMA. 2011;305(22):2304–10.
Lynch HT et al. Review of the Lynch syndrome: history, molecular genetics, screening, differential diagnosis, and medicolegal ramifications. Clin Genet. 2009;76(1):1–18.
Boland CR et al. The biochemical basis of microsatellite instability and abnormal immunohistochemistry and clinical behavior in Lynch syndrome: from bench to bedside. Familial Cancer. 2008;7(1):41–52.
Chang DK et al. Steady-state regulation of the human DNA mismatch repair system. J Biol Chem. 2000;275(24):18424–31.
Kansikas M, Kariola R, Nystrom M. Verification of the three-step model in assessing the pathogenicity of mismatch repair gene variants. Hum Mutat. 2011;32(1):107–15.
Peltomäki P. Epigenetic mechanisms in the pathogenesis of Lynch syndrome. Clin Genet. 2014;85(5):403–12.
Ollikainen M et al. Mechanisms of inactivation of MLH1 in hereditary nonpolyposis colorectal carcinoma: a novel approach. Oncogene. 2007;26(31):4541–9.
Ward RL et al. Identification of constitutional MLH1 epimutations and promoter variants in colorectal cancer patients from the colon cancer family registry. Genet Med. 2013;15(1):25–35.
Ligtenberg MJL et al. Heritable somatic methylation and inactivation of MSH2 in families with Lynch syndrome due to deletion of the 3[prime] exons of TACSTD1. Nat Genet. 2009;41(1):112–7.
Nagasaka T et al. Somatic hypermethylation of MSH2 is a frequent event in Lynch syndrome colorectal cancers. Cancer Res. 2010;70(8):3098–108.
Weisenberger DJ et al. CpG island methylator phenotype underlies sporadic microsatellite instability and is tightly associated with BRAF mutation in colorectal cancer. Nat Genet. 2006;38(7):787–93.
Parsons MT et al. Correlation of tumour BRAF mutations and MLH1 methylation with germline mismatch repair (MMR) gene mutation status: a literature review assessing utility of tumour features for MMR variant classification. J Med Genet. 2012;49(3):151–7.
Rodriguez-Soler M et al. Risk of cancer in cases of suspected lynch syndrome without germline mutation. Gastroenterology. 2013;144(5):926–32. e1; quiz e13-4.
Mensenkamp AR et al. Somatic mutations in MLH1 and MSH2 are a frequent cause of mismatch-repair deficiency in lynch syndrome-like tumors. Gastroenterology. 2014;146(3):643–646.e8. The authors show that two specific somatic mutations account for over half of the cases of MSI in patients without underlying germline mutations or promoter hyper-methylation.
Zhang B et al. Antimetastatic role of Smad4 signaling in colorectal cancer. Gastroenterology. 2010;138(3):969–80. e1-3.
Parsons R et al. Microsatellite instability and mutations of the transforming growth factor beta type II receptor gene in colorectal cancer. Cancer Res. 1995;55(23):5548–50.
Jung B et al. Loss of activin receptor type 2 protein expression in microsatellite unstable colon cancers. Gastroenterology. 2004;126(3):654–9.
Rampino N et al. Somatic frameshift mutations in the BAX gene in colon cancers of the microsatellite mutator phenotype. Science. 1997;275(5302):967–9.
Baba Y. PTGER2 overexpression in colorectal cancer is associated with microsatellite instability, independent of CpG island methylator phenotype. Cancer Epidemiol, Biomarkers Prev: Publ Am Assoc Cancer Res, Am Soc Prev Oncol. 2010;19(3):822–31.
Calin GA et al. Genetic progression in microsatellite instability high (MSI-H) colon cancers correlates with clinico-pathological parameters: a study of the TGRbetaRII, BAX, hMSH3, hMSH6, IGFIIR and BLM genes. Int J Cancer J Int du Cancer. 2000;89(3):230–5.
Nosho K et al. PIK3CA mutation in colorectal cancer: relationship with genetic and epigenetic alterations. Neoplasia. 2008;10(6):534–41.
Nosho K et al. Cyclin D1 is frequently overexpressed in microsatellite unstable colorectal cancer, independent of CpG island methylator phenotype. Histopathology. 2008;53(5):588–98.
Souza RF et al. Microsatellite instability in the insulin-like growth factor II receptor gene in gastrointestinal tumours. Nat Genet. 1996;14(3):255–7.
Smyrk TC et al. Tumor-infiltrating lymphocytes are a marker for microsatellite instability in colorectal carcinoma. Cancer. 2001;91(12):2417–22.
Prall F et al. Prognostic role of CD8+ tumor-infiltrating lymphocytes in stage III colorectal cancer with and without microsatellite instability. Hum Pathol. 2004;35(7):808–16.
Schwitalle Y et al. Immune response against frameshift-induced neopeptides in HNPCC patients and healthy HNPCC mutation carriers. Gastroenterology. 2008;134(4):988–97.
Sherwood AM et al. Tumor-infiltrating lymphocytes in colorectal tumors display a diversity of T cell receptor sequences that differ from the T cells in adjacent mucosal tissue. Cancer Immunol, Immunotherapy: CII. 2013;62(9):1453–61. This article suggests presence of an adaptive immune response within CRC cells that varies in strength and breadth among patients.
Buckowitz A et al. Microsatellite instability in colorectal cancer is associated with local lymphocyte infiltration and low frequency of distant metastases. Br J Cancer. 2005;92(9):1746–53.
Alexander J et al. Histopathological identification of colon cancer with microsatellite instability. Am J Pathol. 2001;158(2):527–35.
Kloor M et al. Clinical significance of microsatellite instability in colorectal cancer. Langenbeck’s archives of surgery / Deutsche Gesellschaft fur Chirurgie. 2014;399(1):23–31.
Jenkins MA et al. Pathology features in Bethesda guidelines predict colorectal cancer microsatellite instability: a population-based study. Gastroenterology. 2007;133(1):48–56.
Thibodeau SN et al. Microsatellite instability in colorectal cancer: different mutator phenotypes and the principal involvement of hMLH1. Cancer Res. 1998;58(8):1713–8.
Popat S, Hubner R, Houlston RS. Systematic review of microsatellite instability and colorectal cancer prognosis. J Clin Oncol. 2005;23(3):609–18.
Sinicrope FA et al. Prognostic impact of microsatellite instability and DNA ploidy in human colon carcinoma patients. Gastroenterology. 2006;131(3):729–37.
Watanabe T et al. Chromosomal instability (CIN) phenotype, CIN high or CIN low, predicts survival for colorectal cancer. J Clin Oncol: Off J Am Soc Clin Oncol. 2012;30(18):2256–64.
Gavin PG et al. Mutation profiling and microsatellite instability in stage II and III colon cancer: an assessment of their prognostic and oxaliplatin predictive value. Clin Cancer Res: Off J Am Assoc Cancer Res. 2012;18(23):6531–41.
Koi M et al. Human chromosome 3 corrects mismatch repair deficiency and microsatellite instability and reduces N-methyl-N’-nitro-N-nitrosoguanidine tolerance in colon tumor cells with homozygous hMLH1 mutation. Cancer Res. 1994;54(16):4308–12.
Carethers JM et al. Competency in mismatch repair prohibits clonal expansion of cancer cells treated with N-methyl-N’-nitro-N-nitrosoguanidine. J Clin Invest. 1996;98(1):199–206.
Hawn MT et al. Evidence for a connection between the mismatch repair system and the G2 cell cycle checkpoint. Cancer Res. 1995;55(17):3721–5.
Carethers JM et al. Mismatch repair proficiency and in vitro response to 5-fluorouracil. Gastroenterology. 1999;117(1):123–31.
Fink D et al. The role of DNA mismatch repair in platinum drug resistance. Cancer Res. 1996;56(21):4881–6.
Aebi S et al. Resistance to cytotoxic drugs in DNA mismatch repair-deficient cells. Clin Cancer Res: Off J Am Assoc Cancer Res. 1997;3(10):1763–7.
Hemminki A et al. Microsatellite instability is a favorable prognostic indicator in patients with colorectal cancer receiving chemotherapy. Gastroenterology. 2000;119(4):921–8.
Elsaleh H et al. Association of tumour site and sex with survival benefit from adjuvant chemotherapy in colorectal cancer. Lancet. 2000;355(9217):1745–50.
Ribic CM et al. Tumor microsatellite-instability status as a predictor of benefit from fluorouracil-based adjuvant chemotherapy for colon cancer. N Engl J Med. 2003;349(3):247–57.
de Vos tot Nederveen Cappel WH et al. Survival after adjuvant 5-FU treatment for stage III colon cancer in hereditary nonpolyposis colorectal cancer. Int J Cancer J Int du Cancer. 2004;109(3):468–71.
Storojeva I et al. Prognostic and predictive relevance of microsatellite instability in colorectal cancer. Oncol Rep. 2005;14(1):241–9.
Benatti P et al. Microsatellite instability and colorectal cancer prognosis. Clin Cancer Res: Off J Am Assoc Cancer Res. 2005;11(23):8332–40.
Lanza G et al. Immunohistochemical test for MLH1 and MSH2 expression predicts clinical outcome in stage II and III colorectal cancer patients. J Clin Oncol: Off J Am Soc Clin Oncol. 2006;24(15):2359–67.
Jover R et al. Mismatch repair status in the prediction of benefit from adjuvant fluorouracil chemotherapy in colorectal cancer. Gut. 2006;55(6):848–55.
Kim GP et al. Prognostic and predictive roles of high-degree microsatellite instability in colon cancer: a national cancer institute-national surgical adjuvant breast and bowel project collaborative study. J Clin Oncol: Off J Am Soc Clin Oncol. 2007;25(7):767–72.
Des Guetz G et al. Does microsatellite instability predict the efficacy of adjuvant chemotherapy in colorectal cancer? A systematic review with meta-analysis. Eur J Cancer. 2009;45(10):1890–6.
Sargent DJ et al. Defective mismatch repair as a predictive marker for lack of efficacy of fluorouracil-based adjuvant therapy in colon cancer. J Clin Oncol. 2010;28(20):3219–26.
Bertagnolli MM et al. Microsatellite instability predicts improved response to adjuvant therapy with irinotecan, fluorouracil, and leucovorin in stage III colon cancer: cancer and leukemia group B protocol 89803. J Clin Oncol: Off J Am Soc Clin Oncol. 2009;27(11):1814–21.
Klingbiel D et al. Prognosis of stage II and III colon cancer treated with adjuvant 5-fluorouracil or FOLFIRI in relation to microsatellite status: results of the PETACC-3 trial. Ann Oncol. 2015;26(1):126–32.
des Guetz G et al. Microsatellite instability and sensitivitiy to FOLFOX treatment in metastatic colorectal cancer. Anticancer Res. 2007;27(4C):2715–9.
Kim ST et al. Clinical impact of microsatellite instability in colon cancer following adjuvant FOLFOX therapy. Cancer Chemother Pharmacol. 2010;66(4):659–67.
Sinicrope FA et al. Prognostic impact of deficient DNA mismatch repair in patients with stage III colon cancer from a randomized trial of FOLFOX-based adjuvant chemotherapy. J Clin Oncol: Off J Am Soc Clin Oncol. 2013;31(29):3664–72. The study demonstrates differences in prognosis for stage III MMR-deficient CRC based on tumor site and degree of lymph node involvement.
Le DT et al. PD-1 blockade in tumors with mismatch-repair deficiency. New England Journal of Medicine, 2015. The article provides evidence for efficacy of immune checkpoint blockade in the treatment of metastastic, MMR-deficient CRC.
Compliance with Ethics Guidelines
Conflict of Interest
Neil Majithia declares that he has no conflict of interest.
Benjamin R. Kipp has received support through a grant from Abbott Molecular, Inc.
Axel Grothey has received support through research grants to the Mayo Foundation from Genentech, Bayer, Eisai, BBI, and Eli Lilly.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Translational Colorectal Oncology
Rights and permissions
About this article
Cite this article
Majithia, N., Kipp, B.R. & Grothey, A. Distinctive Tumor Biology of MSI-High Colorectal Cancer. Curr Colorectal Cancer Rep 11, 281–287 (2015). https://doi.org/10.1007/s11888-015-0283-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11888-015-0283-4