Abstract
Purpose
CMV infection remains a priority for vaccine development. Vaccination of infants could modify congenital infection and provide lifetime immunity. Properties of CMV-specific T cells associated with control of viral replication in early life have not been fully defined.
Methods
CMV-specific CD4 and CD8 T cell responses were investigated in infants with congenital CMV infection and compared to adults with primary or chronic infection. PBMC were stimulated with UL83 (pp65) or UL122 (IE-2) peptide pools then stained with antibodies to markers of T cell subset (CD4 or CD8), phenotype (CD45RA, CCR7), or function (MIP1β, CD107, IFNγ, IL2) for flow cytometry analysis.
Results
Detection of CMV pp65-specific CD4 T cells was less common in infants than adults. Responder cells were primarily effector memory (EM, CD45RA-CCR7-) in adults, but mixed memory subsets in infants. Detection of CMV pp65-specific CD8 T cells did not differ between the groups, but infants had lower frequencies of total responding cells and of MIP1β- or CD107-expressing cells. Responder cells were EM or effector memory RA (CD45RA + CCR7-) in all groups. Polyfunctional T cells were less commonly detected in infants than adults. Responses to IE-2 were detected in adults but not infants. All infants had detectable circulating CMV DNA at initial study (versus 60 % of adults with primary infection) despite longer duration of CMV infection.
Conclusions
Reduced frequencies and altered functional profile of CMV-specific CD4 and CD8 T cell responses were detected in infants compared to adults, and were associated with persistent CMV DNA in peripheral blood.
Similar content being viewed by others
Introduction
Congenital cytomegalovirus (CMV) infection is a significant cause of infant morbidity, and remains a high priority for vaccine development [1]. An estimated 0.7 % of live births or 30,000 infants per year in the U.S. are diagnosed with congenital CMV infection, and nearly 20 % exhibit permanent neurologic disabilities [2]. Moreover, children with congenital or postnatal CMV infection may shed virus in the urine for prolonged periods, increasing the risk of primary CMV infection in their seronegative pregnant caretakers [3–5]. One possible CMV vaccine strategy is to immunize infants to prevent CMV infection, reduce viral shedding after primary infection, or to reduce the risk of severe congenital infection during pregnancy later in life, which has been a rationale for universal rubella immunization [6–8]. Another strategy is to vaccinate infants with congenital CMV infection as immunotherapy with or without antiviral agents, which has been the basis of CMV vaccine clinical trials in seropositive women or stem cell transplant recipients [6, 9, 10]. Such approaches require that the candidate vaccine induce potent CMV-specific neutralizing antibodies and/or cell-mediated immune responses in infants that correlate with control of viral replication or protection from disease, persist in long-term memory, and are evaluable in clinical trials [11–13]. Moreover, these responses must continuously adapt to the large diversity and rapid evolution of CMV populations within distinct host tissue compartments [14].
The generation and maintenance of anti-viral T cell responses over the course of CMV infection, and their role in protection from severe clinical disease or control of viral replication have not been fully defined [15]. Virus-specific T cells with distinct antigen specificity, functional capacity, or surface phenotype have been shown to affect viral disease pathogenesis in a variety of models, including animals [16, 17] or adult humans [18–21]. However, unique features of cellular immunity in young children may affect their ability to generate protective anti-viral T cell responses during primary infection or following vaccination, and therefore warrant further investigation in longitudinal studies [7, 11]. Congenital CMV infection is a model system to characterize these T cells. Prior work by our group and others [22–28] suggests that healthy infants and young children can generate CMV-specific cell-mediated immune responses, but a detailed analysis of their phenotype and function has not been performed.
Efforts to define immune correlates of protective anti-viral T cell responses in adults have focused extensively on memory phenotype and effector function. Among other markers, antigen-experienced T cells can be distinguished by expression patterns of the transmembrane phosphatase CD45 isoform and the lymph node homing molecule CCR7 [29]. Moreover, increasing evidence suggests that T cells capable of multiple simultaneous anti-viral effector functions are associated with markers of protection [30–32], and that analysis of these polyfunctional memory T cells may be used to evaluate outcome following vaccination [13, 33–36].
Our aim was to characterize CMV-specific T cell memory phenotype and effector functions in young infants with congenital CMV infection compared to adults with primary or chronic infection, and to correlate these responses with longitudinal viral load measurements. This identifiable infant population was utilized as a model of longitudinal CMV-specific cellular immune responses to provide a foundation for studies in healthy infants with primary post-natal CMV infection, a more difficult population to identify. Using multi-parameter flow cytometry, we demonstrate quantitative and qualitative differences in CMV-specific CD4 and CD8 T cell responses in infants compared to adults.
Methods
Study Population
Ten infants with congenital CMV infection were studied longitudinally. They were enrolled at the University of Massachusetts Medical Center (Worcester, MA), Baystate Children’s Hospital (Springfield, MA), and Policlinico San Matteo (Pavia, Italy). Diagnosis of congenital CMV infection was performed within 3 weeks of birth by detection of CMV DNA in neonatal blood [37, 38] and/or virus isolation from urine. Three of 10 infants were symptomatic with central nervous system (CNS) involvement.
Ten pregnant women with primary CMV infection were studied longitudinally as adult controls for primary CMV infection. They were enrolled at Policlinico San Matteo (Pavia, Italy). Diagnosis of primary CMV infection was based on one or more of the following criteria: recent CMV-specific IgG seroconversion, presence of CMV-specific IgM and low IgG avidity, and/or presence of CMV nucleic acids in blood [39]. Timing of primary CMV infection was based on decreasing levels of CMV-specific IgM antibody, increasing levels of IgG avidity, presence of clinical symptoms, and/or laboratory findings [40]. Healthy infants with no CMV or HIV infection who were born to HIV-1-infected women [24], and healthy adults with chronic or no CMV infection, served as additional controls. Pregnant women and HIV-uninfected infants of HIV-infected women have been shown to generate robust CMV- or vaccine-specific cellular immune responses, respectively [41–43]. Infants and non-pregnant adults with primary CMV infection were not available as controls for this study. Infection is typically asymptomatic or mild in these populations, so they are infrequently identified by health care providers and therefore rarely available for study enrollment.
These studies were approved by human subjects committees at participating institutions. Written informed consent was obtained from adult participants or from a parent or legal guardian of infants.
Peripheral Blood Mononuclear Cell Stimulation and Staining
Peripheral blood mononuclear cells (PBMC) were processed as described [24] and were stimulated (0.5 × 106 in 250 μl RPMI with 10 % fetal calf serum) with pools of overlapping peptides spanning CMV UL83 (pp65) or UL122 (immediate early (IE)-2). Anti-CD107a and -CD107b (Alexa-647), brefeldin A, and monensin (BD Pharmingen) and antibodies to co-stimulatory molecules CD28 and 49d were added with peptide. Following a 6-h incubation, cells were fixed, stained with antibodies specific for CD8 (ECD), CD4 (Pacific Blue), CD14/16/19 (APC-Cy7), CD45RA (FITC), CCR7 (PE-Cy7), and vital stain Live/Dead Blue (Invitrogen), permeabilized, then stained with macrophage inflammatory protein (MIP)-1β (PE), interferon (IFN)-γ (Alexa-700), and interleukin (IL)-2 (PerCP-Cy5.5). All antibodies were obtained in the conjugated form from BD Pharmingen with the exception of CD107a/b, CD14/16/19, IFNγ, and IL2 obtained from BioLegend. Medium alone and staphylococcus enterotoxin B (SEB, Toxin Technology, Sarasota, FL) were used as negative and positive controls, respectively. Longitudinal samples from each subject were studied simultaneously in the same assay.
CMV Peptides
The CMV pp65 peptide pool (15 amino acid peptides overlapping by 11 amino acids) was obtained through the NIH AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH. The CMV IE-2 peptide pool was synthesized as described [44].
Flow Cytometry
Flow cytometry was performed using a LSRII instrument (BD Bioscience, San Jose, CA) and data were analyzed using FlowJo (TreeStar, Palo Alto, CA), Pestle (version 1.6.2 Mario Roederer, VRC, NIAID, NIH, Bethesda, MD), or SPICE (version 5, Mario Roederer, VRC, NIAID, NIH) software.
Cells of interest were identified by a serial gating algorithm incorporating lymphocytes (forward versus side scatter), cell singlets, live cells, CD14/16/19- cells (to enrich for CD3 cells), CD4+ or CD8+ cells, and memory cells (CD45RA or CCR7). Thresholds to define negative CD45RA or CCR7 cell populations were determined using “fluorescence minus one” tubes that included all antibodies in the panel except CD45RA or CCR7, respectively.
CD4 or CD8 T cells were distinguished by patterns of CD45RA or CCR7 expression, and included naïve (CD45RA+CCR7+) and total memory T cell populations. Total memory T cells were further divided into subsets defined as central memory (CM, CD45RA-CCR7+), effector memory (EM, CD45RA-CCR7-), or effector memory RA (EMRA, CD45RA+CCR7-) [29]. Frequencies of total memory T cells were calculated as a percentage of all CD4 or CD8 T cells, and memory T cell subsets (CM, EM, or EMRA) were calculated as a percentage of total memory T cells, where total = (CM + EM + EMRA) (Fig. 1).
Gating strategy for memory T cell populations. Representative dot plots with memory T cells populations for a mother (A110) and her infant (P110) are shown. CD4 (left column) or CD8 (right column) T cells are differentiated by CD45RA (y-axis) or CCR7 (x-axis) expression. T cell populations include total memory (L-shaped gate incorporating CM + EM + EMRA as indicated in legend diagram) with subsets central memory (CM, CC45RA-CCR7+, lower right quadrant), effector memory (EM, CD45RA-CCR7-, lower left quadrant), or effector memory RA (EMRA, CD45RA + CCR7-, upper left quadrant). Naïve T cells (CD45RA+CCR7+, upper right quadrant) are not included in the gate
CD4 or CD8 T cell functional responses were calculated by subtracting the frequencies of response without stimulation from the response with CMV or SEB stimulation (“background-subtracted”). The threshold to define “detectable” response was set at ≥90th percentile of the distribution of negative background-subtracted values [34]. For subjects with 2 or more detectable responses at 1 or more time points, data generated from FlowJo for each functional marker was formatted in Pestle and analyzed for patterns of polyfunctionality using SPICE. As above, the threshold in SPICE was set at ≥90th percentile of the distribution of negative background-subtracted values.
CMV Detection
CMV DNA was quantified in peripheral blood at Policlinico San Matteo (Pavia, Italy) for all subjects. Until December 2007, samples were tested initially by quantitative PCR with detection limit 10 genome equivalents (GE)/10 μl whole blood. Samples negative for DNA were then tested in triplicate by nested PCR [39, 45]. Samples DNA negative by quantitative PCR and positive by nested PCR (i.e., DNA present but <10 GE/10 μl) are reported as 3 GE/10 μl. After December 2007, samples were tested by real-time PCR with detection limit 25 GE/ml whole blood) [46]. The two assays were internally calibrated and validated by an external quality control program (Quality Control for Molecular Diagnostics, www.qcmd.org). Clinical laboratories at participating sites performed virus isolation from urine.
Data Analysis
Nonparametric Savage scores were used to compare the percentages of total memory T cells and of memory T cell subsets (CM, EM, or EMRA) at initial study, presence of detectable CMV-specific T cell responses at any time, frequency of CMV pp65-specific T cells at initial study, and presence of polyfunctional responses at any time between all study groups. Nonparametric Wilcoxon Rank-Sum tests were used to compare percentages of CMV pp65-specific memory T cell subsets between C infants and P adults, CMV viral load at initial study, and duration of detectable CMV DNAemia. Fisher’s Exact Test was used to compare detectable CMV DNA in the peripheral blood at initial study between C infants and P adults. SAS v 9.3 software was used for all of these analyses (SAS Institute, Inc., Cary, North Carolina).
Wilcoxon Rank-Sum Test was used to compare the frequencies of polyfunctional responses. Normalized data is shown, i.e., each measurement value is weighted by its relative contribution to the total of all measurements for the sample, and expressed as “percent of total response” [47]. Statistical significance was defined as p < 0.05.
Results
Study Population
Table 1 shows characteristics of the study population. Study subjects were infants with congenital (C) CMV infection. Control subjects were infants with no (N) CMV infection and adults with primary (P), chronic (CH), or no (N) CMV infection. All but 3 infants with congenital CMV infection (“C infants”), and all adults with primary CMV infection (“P adults”), were studied at ≥2 time points. Infants with no CMV infection (“N infants”) were studied at 2 time points (2–6 months and 12–18 months of age). Adults with chronic (“CH adults”) or no (“N adults”) CMV infection were each studied at 1 time point.
For P adults, median gestational age at onset of primary infection was 8 weeks (range 0 to 21 weeks). Median duration of CMV infection at initial study, defined as the time between onset and initial study, was 9 weeks (range 3 to 13 weeks).
For C infants, median postnatal age at initial study was 4 weeks (range 2 days to 30 weeks). Median duration of CMV infection at initial study, defined as time between onset of maternal infection and infant initial study, was 32 weeks (range 22 to 63 weeks) for 6 infants with known timing of maternal infection. This definition likely overestimates the duration of CMV infection at initial study by approximately 6 weeks, during which time transmission from mother to fetus occurs [48]. Samples obtained in utero during early CMV infection were not available.
Memory T Cell Subsets in Infants and Adults
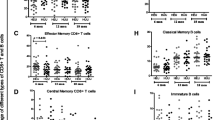
We first compared the frequencies of total memory and memory subset CD4 and CD8 T cell populations between all cohort groups (Fig. 2). The median frequencies of total memory T cells were significantly lower in C infants compared to P adults (CD4 30 % versus 66 %, p = 0.003; CD8 40 % versus 71 %, p = 0.022), and in N infants compared to N adults (CD4 33 % versus 74 %, p = 0.002; CD8 29 % versus 77 %, p = 0.002). The median frequencies of total memory CD4 T cells in C and N infants were not significantly different.
Median frequencies of memory T cells at initial study. Total memory T cells (full bars; CD4 left panel; CD8 right panel) are shown as percent of all (total memory plus naïve) T cells, and memory T cell subsets (regions within full bars as noted in legend) are shown as percent of total memory T cells. Compared to P adults, C infants had lower total memory CD4 (p = 0.003) or CD8 (p = 0.022) T cell frequencies. Compared to N adults, N infants had lower total memory CD4 (p = 0.002) or CD8 (p = 0.002) T cell frequencies. There were no significant differences in frequencies of total memory T cells between P, CH, and N adult groups. See text for comparison of T cell subsets. C, congenital infant; P, primary adult; CH, chronic adult; N (I), no infection infant; N (A), no infection adult; EM, effector memory (CD45RA-CCR7-); CM, central memory (CC45RA-CCR7+); EMRA, effector memory RA (CD45RA+CCR7-)
For both C and N infant groups, the majority of memory CD4 T cells were CD45RA-CCR7+ (CM; median frequencies > 51 %; p < 0.05 versus all adult groups), and the majority of memory CD8 T cells were CD45RA+CCR7- (EMRA; median frequencies > 60 %; p < 0.02 versus all adult groups). In contrast, for all P, CH, and N adult groups, the majority of memory CD4 or CD8 T cells were CD45RA-CCR7- (EM; median frequencies > 58 %, p < 0.01 versus both infant groups).
The median frequencies of EM T cells were significantly lower for C infants compared to P adults (CD4 23 % versus 52 %, p = 0.003; CD8 29 % versus 65 %, p = 0.003) and for N infants compared to N adults (CD4 25 % versus 50 %, p = 0.002; CD8 12 % versus 59 %, p = 0.002). Conversely, the median frequencies of EMRA T cells were significantly higher for C infants compared to P adults (CD4 8 % versus 6 %, p = 0.046; CD8 63 % versus 33 %, p = 0.007) and for N infants compared to N adults (CD4 17 % versus 10 %, p = 0.013; CD8 80 % versus 40 %, p = 0.006). The median frequencies of CM CD4 T cells were significantly higher for C infants compared to P adults (68 % versus 42 %, p = 0.036), and for N infants compared to N adults (51 % versus 43 % at p = 0.05). CM CD8 T cells were negligible for all groups. The frequencies of total or subset memory T cell populations in P, CH, and N adults did not differ significantly.
Altered Function and Phenotype of CMV pp65-Specific T Cell Responses in Infants
To quantify the frequencies and characterize the functional properties of CMV-specific T cells in infants and adults at distinct stages of infection, we compared CD4 or CD8 T cell responses to CMV pp65 or IE-2 peptide pools measured by markers of cytotoxicity (CD107), chemotaxis (MIP1β), or anti-viral cytokine secretion (IFNγ or IL2). Both CMV gene products are common targets for CMV-specific T cell responses in adults with chronic infection [49]. In particular, CD4 T cells targeted IE-2 more frequently than IE-1 in adults, so we chose to examine IE-2 in infants rather than IE-1 as in our previous studies [23, 24]. Representative flow cytometry plots for a mother-infant pair show CD4 T cell responses to CMV pp65 or SEB as measured by CD107, MIP1β, IFNγ, or IL2 expression (Fig. 3). Responses to IE-2 were not detected in any C infants, but were detected in 1 of 10 (CD4 only) P adults and 2 of 5 (CD8 only) CH adults (data not shown). We therefore focused on characterizing responses to CMV pp65. Overall, no distinct longitudinal trend of pp65-specific CD4 or CD8 T cell responses was observed for any group, except P adults showed no IL2 expression by pp65-specific CD4 T cells more than 3 months after onset of infection (data not shown).
For pp65-specific CD4 T cells, a lower proportion (2 of 10; p = 0.019) of C infants had detectable responses by any functional marker at any time point compared to P adults (Fig. 4). In these 2 C infants, single IFNγ- and single IL2-expressing CD4 T cells were detected at only 1 (6 months or 19 months, respectively) of 5 time points over the first 15 or 19 months of age, respectively (Fig. 5). CMV pp65-specific CD4 T cells were found primarily within the EM T cell subset for P adults, but were distributed fairly equally across subsets for the 2 C infants with responses (Fig. 6). The median proportion of EM cells was lower (p = 0.03) and of EMRA cells was higher (p = 0.04) for C infants compared to P adults.
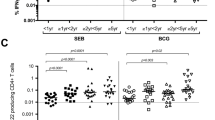
Detectable CMV pp65-specific T cell responses. Percentages of infants or adults with detectable CMV pp65-specific CD4 (left) or CD8 (right) T cell responses by any measure of function at any time point are shown. All comparisons are not significantly different except CD4 T cells for C infants versus P adults (p = 0.019)
Frequencies of detectable CMV pp65-specific T cell responses. Box plots display the frequencies of CMV pp65-specific CD4 (left) or CD8 (right) T cells detected by individual measures of function (CD107, MIP1β, IFNγ, or IL2) for C infants, P adults, and CH adults. All detectable responses and timepoints are included. Horizontal lines represent the 25th, 50th (median), or 75th percentiles. Diamonds indicate the mean frequencies, and the error bars indicate the minimum and maximum values. C infants showed significantly lower frequencies of pp65-specific CD8 T cells detectable by any function (i.e., gated on all responding cells, not shown; p = 0.006 versus P adults; p = 0.040 versus CH adults) or by MIP1β (p = 0.043 versus P adults; p = NS versus CH adults). Frequencies of responses measured by CD107, IFNγ, or IL2 were not significantly different between the groups
CMV pp65-specific memory T cell subsets. Frequencies of memory T cell subsets (CM, EM, or EMRA) for responding CMV pp65-specific CD4 (left) or CD8 (right) T cells are shown. All detectable responses and time points are included. Median frequencies are indicated by horizontal bars. CMV pp65-specific CD4 T cells were found primarily within the EM T cell subset for P adults, but were distributed relatively equally across subsets for the 2 C infants with responses. These C infants had lower EM (p = 0.03) and higher EMRA (p = 0.04) CD4 T cell frequencies compared to P adults. CMV pp65-specific CD8 T cells were found almost exclusively within the EM or EMRA T cell subsets for all groups
For pp65-specific CD8 T cells, the proportion of C infants and P adults with detectable responses by any functional marker at any time point did not differ (8 of 10 for both groups) (Fig. 4). However, the frequencies of all responding pp65-specific CD8 T cells (p = 0.006 versus P adults; p = 0.040 versus CH adults; data not shown), and of MIP1β- (p = 0.043 versus P adults; p = not significant (NS) versus CH adults) or CD107-expressing (p = NS versus P adults; p = NS versus CH adults) pp65-specific CD8 T cells, were lower in C infants (Fig. 5). Frequencies of responses measured by IFNγ were not significantly different between C, P, and CH groups, and responses by any measure were not significantly different between the P and CH adult groups. Responses measured by IL2 were detected in 1 of 5 CH adults but no other subjects. CMV pp65-specific CD8 T cells were found almost exclusively within the EM or EMRA T cell subsets for both C infants and P adults (Fig. 6).
CMV-specific T cell responses were compared between infants with (CNS; n = 3) or without (non-CNS; n = 7) symptomatic infection involving the central nervous system, although no significant differences were identified. Two of 3 CNS infants had detectable CD4 (versus 0 of 7 non-CNS; p = NS) and CD8 (versus 6 of 7 non-CNS; p = NS) T cell responses. Of the CNS infants with detectable CD8 T cells, 0 of 2 (versus 2 of 6 non-CNS; p = NS) had detectable polyfunctional responses.
Reduced Frequencies of Polyfunctional CMV-Specific T Cell Responses in Infants
T cells capable of multiple simultaneous effector functions (“polyfunctional”) have been associated with protection in viral infection [30–32]. To characterize these responses in the study cohorts, we compared the frequencies and patterns of polyfunctional CMV pp65-specific T cells using SPICE analysis for subjects with ≥ 2 functions detected at any time point. Significantly fewer C infants with detectable pp65-specific T cells had polyfunctional responses (0 of 2 with CD4 responses; 2 of 8 with CD8 responses) compared to P (CD4 p = 0.033; CD8 p = 0.003) or CH (CD8 p = 0.011) adults (Fig. 7). Patterns of CD4 T cell functions varied for P and CH adults, but IL2 secretion was the least common (Fig. 8). Fewer C infants had polyfunctional CD8 T cells than adults (p < 0.05), but when present followed the most common patterns of adults with co-expression of CD107/IFNγ/MIP1β or of CD107/MIP1β (Fig. 8). Fewer C infants had single expression of CD107 than adults (p < 0.05).
Detectable polyfunctional T cell responses. Percentages of subjects with detectable polyfunctional CMV pp65-specific CD4 (left) or CD8 (right) T cells by any combination of ≥ 2 functions at any time point are shown. Significantly fewer C infants had polyfunctional CD4 or CD8 T cells compared to P or CH adults. The 2 C infants with detectable CD4 T cell responses did not have polyfunctional cells
Patterns of polyfunctional T cell responses. Polyfunctional CMV pp65-specific CD4 (top panel) or CD8 (bottom panel) T cell responses were defined as expression of ≥2 measures of function (CD107, MIP1β, IFNγ, and/or IL2) on stimulation with CMV pp65. Normalized data is shown, i.e. each measurement value (frequency of response) is weighted by its relative contribution to the total of all measurements in the group (C, P, or CH), and expressed as “percent of total response” for each group. Pies (legend top) illustrate relative frequencies of responses with 1–4 functions for each group. Bars (legend bottom) represent relative frequencies of responses consisting of the specific functional profiles indicated along the x-axis (boxes). A significantly lower (* p < 0.05) proportion of C infants had polyfunctional CMV pp65-specific CD8 T cells compared to P or CH adults, but when present these cells expressed the same common profiles as adults with co-expression of CD107 and MIP1β with or without IFNγ. In addition, a significantly lower (+ p < 0.05) proportion of C infants had CMV pp65-specific CD8 T cells that expressed CD107 alone compared to P or CH adults
Persistence of Detectable CMV DNA in Peripheral Blood of Infants with Congenital CMV Infection
CMV DNA was detectable in the peripheral blood of all 9 C infants tested, with a median CMV load at initial study of 10 GE/10 μl blood (range 3 to 1000 GE) (Table 1). The median time between onset of maternal infection and infant initial study (a measure of duration of infant CMV infection at initial study) was 26 weeks (range 22 to 63 weeks) for 5 of the 9 C infants (timing of maternal infection was not known for 4 infants).
CMV DNA was detectable in 6 of 10 P adults (p = NS versus C infants), with a median CMV load at initial study of 10 GE/10 μl blood (range 3 to 100 GE; p = NS versus C infants). Median time between onset of infection and initial study (a measure of duration of CMV infection at initial study) for these 6 subjects was 5 weeks (range 3–13 weeks; p = 0.013 versus C infants). CMV DNA was undetectable in the other 4 of 10 P adults at initial study and over the course of the study period. Median time between onset of infection and initial study for these 4 subjects was 11 weeks (range 7–12 weeks; p = 0.037 versus C infants, and p = NS versus 6 P adults with detectable CMV DNA at initial study as above). The median CMV load at initial study for all 10 P adults was 3 GE/10 μl blood (range 0 to 100 GE; p = NS versus C infants) (Table 1).
Discussion
Congenital CMV infection is a significant clinical problem with limited effective prevention or treatment strategies, and serves as a relevant model in which to examine the dynamic lifelong interaction between virus and host. While many studies have demonstrated the critical role of CMV-specific cellular immune responses in controlling viral replication and disease severity, the specific features of these responses that confer protection remain incompletely defined [15]. As a result, development of a CMV vaccine has been relatively empiric with measurable but limited efficacy. The optimal immunization strategy has been shown to include infants less than 1 year of age [6, 50, 51]. To focus these efforts toward identifying the mechanisms of protective cellular immunity in this population, we tested the hypothesis that the memory phenotype or functional capacity of CMV-specific T cells is different and associated with reduced control of viral replication in infants with congenital CMV infection compared to immunocompetent adults with primary or chronic infection. Our data demonstrate reduced or altered CMV pp65-specific CD4, CD8, or poyfunctional T cells in these infants, which were associated with prolonged detection of CMV DNA in the peripheral blood.
We first examined the distribution of total memory CD4 and CD8 T cell populations. CD45RA and CCR7 have been used to identify T cell populations with distinct tissue homing, capacity for rapid proliferation, or anti-viral effector function [29]. Compared to adults, infants had significantly smaller total memory T cell compartments at initial study, primarily due to lower frequencies of the CD45RA-CCR7- EM T cell subset. This pattern was observed regardless of CMV infection status, suggesting an age- rather than virus-specific phenomenon associated with less cumulative antigen exposure in infants compared to adults. The distribution of memory T cell subsets observed in our study is similar to that reported in other cohorts of young healthy children [7, 52].
Consistent with the effect of antigen exposure on the evolution of memory T cell populations, the only significant difference observed at initial study for infants with congenital CMV infection compared to CMV-uninfected infants was higher frequency of EM CD8 T cells. This finding concurs with previous studies showing that congenital viral infection shifts the overall CD8 T cell population to a more differentiated memory phenotype [25, 52]. The clinical significance of relatively late-differentiated CD8 T cells in infants with congenital CMV or other viral infection is not known, but may be associated with such disparate effects as inadequate long-lived protective T cell memory and ineffective control of viral infection over time [53], T cell senescence as observed in CMV-infected older adults [54], or reversing the relative anti-inflammatory nature of neonatal T cell responses [7].
Among immunocompetent adults, heterogeneous and robust CMV-specific CD4 T cells are readily detectable, and have been associated with control of viral replication, less severe disease, and lower risk of mother-to-child viral transmission [19, 55–57]. These cells exhibit higher cytotoxic (CD107) and chemotaxis (MIP1β) and lower helper (IL2) functions, and more advanced stages of maturation [29, 58]. Consistent with this pattern, the majority of adults in our study with primary (7 of 10) or chronic (3 of 5) CMV infection had CMV pp65-specific CD4 T cell responses with various combinations of the 4 functions expressed almost exclusively by EM T cells. In contrast, these responses were rarely detected in infants with congenital CMV infection, even when an expanded panel of functions including IFNγ, IL2, MIP1β, and CD107 was examined using flow cytometry. Only 2 infants had detectable responses, which consisted of IFNγ- or IL2-expressing cells equally distributed within the EM, CM, and EMRA memory T cell compartments. Of interest, these infants were 2 of 3 in the study with symptomatic infection involving the central nervous system.
Our findings concur with other studies of CMV-specific CD4 T cell responses in neonates or young children. Early studies using bulk lymphoproliferative assays showed that responses were uncommon in children with congenital [59–61] or postnatal [62] CMV infection. Of note, Pass et al. [60, 62] demonstrated fewer responses to CMV compared to herpes simplex virus in infants infected with both viruses, suggesting that reduced cellular immunity may be specific for CMV but not other herpesviruses. More recent studies using assays to measure single-cell responses in children with postnatal CMV infection have also shown lower frequencies of CMV-specific CD4 T cells detected by IFNγ or IL2 secretion compared to adults [26–28]. Consistent with our studies, Lidehall et al. recently reported that fewer CMV-specific IFNγ-producing CD4 T cells were detectable in infants with congenital or post-natal CMV infection compared to adults with primary CMV infection [28].
Among adults, CMV pp65-specific CD8 T cells are skewed toward robust cytotoxic and chemotaxis functions, a profile consistent with effector responses and the capacity to recruit cells to local sites of viral replication [63, 64]. In particular, Kim et al. [63] showed that late memory (CD27-CD45RA+) CMV pp65-specific CD8 T cells in healthy adults produce little IL2 but abundant MIP1β. While CMV pp65-specific CD8 T cell responses were detected in most C infants and P adults in our study, the frequencies of these responses, particularly single MIP1β- or CD107-expressing and polyfunctional cells, were lower in infants. In adults, polyfunctional T cells are associated with anti-viral protection measured by slower disease progression, more frequent control of viral replication, or reduced antiviral drug treatment [30–32], and measurement of these responses has been used to evaluate anti-viral vaccine strategies [13, 33–36]. Few studies have examined polyfunctional CD4 or CD8 T cell responses in infants, which were detected infrequently in congenital HIV infection [65, 66], but commonly and with heterogeneous phenotype and functional profiles following Bacillus Calmette-Guérin vaccination [35, 67]. Similar to these HIV studies, our work shows that infants with congenital CMV infection are relatively incapable of generating polyfunctional T cell responses, but when present, expression patterns were similar to adults and included CD107 and MIP1β with or without IFNγ [64].
With these profiles of CMV pp65-specific T cell responses, we then examined the level and duration of detectable CMV DNA in peripheral blood of infants with congenital and adults with primary CMV infection. CMV DNA at initial study was detectable in all C infants compared to only 60 % of P adults, despite significantly longer estimated duration of CMV infection in infants at the time of sampling. While decay rates could not be estimated from our data, duration of detectable CMV DNA in peripheral blood of infants with congenital CMV infection was at least twice as long as adults with primary CMV infection. This finding suggests that infants have more prolonged exposure to viral replication, and that their CMV-specific T cell responses do not result in clearance of viremia. The relationship between level of viral exposure and clinical outcome of congenital CMV infection has not been fully defined, but an association between high viral burden and severe sequelae has been observed in many studies, and has been used as rationale for antiviral treatment of congenital CMV infection [68, 69].
The mechanisms regulating the induction, expansion, differentiation, quality, and maintenance of virus-specific cellular immune responses are not well understood, especially for infants with congenital viral infection, although knowledge in this area is increasing [7, 11]. In addition to engagement of the T cell receptor (TCR) and co-stimulatory ligands, neonatal T cells have been shown to require a sufficient cytokine signal to allow an effective transition from naïve to effector T cells [70]. IL-12 has been identified as a critical mediator in this pathway, and is required to sustain phosphorylation and expression of proximal TCR signal transduction proteins CD3ξ and Lck. However, IL-12 production by antigen-presenting cells (APC) is impaired through at least the first year of life, which likely limits the capacity of infants to generate adequate virus-specific T cell responses that control viral replication and limit clinical disease. Our data show that infants are relatively incapable of generating polyfunctional CD8 T cell responses but when present expression patterns are similar to adults, thus supporting the model of a critical induction threshold for induction of effector T cell responses.
Moreover, infants characteristically exhibit cytokine profiles favoring polarization of T cells to relatively more anti- (especially T helper 2 and regulatory T cells) than pro-inflammatory responses [71]. In turn, limited CD4 T cell help and/or amplified T cell suppression may lead to suboptimal generation or maintenance of virus-specific CD8 T cells. In humans and animal models, CD8 T cells generated with inadequate CD4 T cell help during infection or vaccination fail to sustain anti-viral effector function or protection [13, 36, 72]. In addition, T cell functional impairment or “exhaustion” is a distinct molecular state associated with chronic viral infection, which involves expression of inhibitory co-receptors such as programmed death receptor-1 (PD1) and cytotoxic T-lymphocyte antigen 4 (CTLA4), reduced effector functions and proliferative capacity, and relatively terminal differentiation, and correlates with markers of disease progression [53]. These T cells have been identified in primary CMV infection [73]. Similarly, our data showing relatively differentiated CD8 T cells in infants with compared to those without congenital CMV infection raises the possibility that these cells are in a state of functional impairment. At the same time, this state may limit T cell-mediated pathologic inflammation and tissue damage [53], especially in the central nervous system that can lead to severe neurodevelopmental delay in some children with congenital CMV infection. While our study did not identify a difference in detectable CD4 or CD8 T cell responses in CNS compared to non-CNS affected infants due to the small cohort, others have shown evidence of immunopathology in mouse [74] and human fetus [75] models. These and other mechanisms intrinsic to or affecting the developing immune system, along with viral immune evasion [76] or genomic evolution [14], perturb the dynamic virus-host interaction to favor persistent viral replication or severe disease in early life.
Conclusions
Our study provides new knowledge of neonatal anti-viral cellular immune responses, and supports ongoing efforts to delineate relevant mechanisms of protection and to develop an effective preventive or therapeutic CMV vaccine targeting young children. Using an expanded flow cytometry panel of functional markers, we show that CMV-specific T cell responses can be primed in early life, but their frequency or “quality” may be suboptimal for controlling CMV infection. Compared to adults, infants with congenital CMV infection have less frequently detectable CMV pp65-specific CD4 T cells and lower frequencies of CD8 T cells capable of cytotoxic, chemotaxis, and multiple simultaneous functions, all in the setting of persistent detectable CMV DNA in the peripheral blood. Moreover, recent studies show that CMV genome populations are highly variable between tissue compartments [14], necessitating plasticity of immune responses within the host. The implication of these findings is that an effective CMV vaccine targeting young children would need to accommodate specific features of adaptive immunity in this age group. Toward this end, a recent report showed that a vaccine based on the CMV gH/gL-pentamer complex induces robust humoral responses that neutralize CMV infection of endothelial cells and fibroblasts [12]. This candidate vaccine may overcome the limitations of the neonatal immune system, especially if combined with pp65, IE1/2, or other targets of cellular responses [44].
Our work demonstrates the feasibility of novel experimental approaches applied to studies of infants that can be used for evaluation of this population in clinical trials. Moreover, our work provides a foundation from which to study CMV-specific cellular immunity in healthy infants with primary or chronic CMV infection, a more difficult population to identify. The phenotypic and functional profiles of protective anti-viral T cells that persist into memory are not fully defined for any population [15, 29], so further characterization of these features will be particularly critical to the design of CMV prevention and treatment strategies.
References
Stratton KR, Durch JS, Lawrence RS. Vaccines for the 21st Century: A Tool for Decisionmaking. Available at: http://www.nap.edu/catalog.php?record_id=5501. Stratton KR, Durch JS, Lawrence RS, editors. Washington, DC: National Academies Press; 2000.
Cannon MJ. Congenital cytomegalovirus (CMV) epidemiology and awareness. J Clin Virol. 2009;46 Suppl 4:S6–10.
Adler SP. Cytomegalovirus and child day care. Evidence for an increased infection rate among day-care workers. N Engl J Med. 1989;321(19):1290–6.
Adler SP. Cytomegalovirus and child day care: risk factors for maternal infection. Pediatr Infect Dis J. 1991;10(8):590–4.
Noyola DE, Demmler GJ, Williamson WD, Griesser C, Sellers S, Llorente A, et al. Cytomegalovirus urinary excretion and long term outcome in children with congenital cytomegalovirus infection. Congenital CMV Longitudinal Study Group. Pediatr Infect Dis J. 2000;19(6):505–10.
Krause PR, Bialek SR, Boppana SB, Griffiths PD, Laughlin CA, Ljungman P, et al. Priorities for CMV vaccine development. Vaccine. 2013.
Prendergast AJ, Klenerman P, Goulder PJ. The impact of differential antiviral immunity in children and adults. Nat Rev Immunol. 2012;12(9):636–48.
Lanzieri TM, Bialek SR, Ortega-Sanchez IR, Gambhir M. Modeling the potential impact of vaccination on the epidemiology of congenital cytomegalovirus infection. Vaccine. 2014.
Kharfan-Dabaja MA, Boeckh M, Wilck MB, Langston AA, Chu AH, Wloch MK, et al. A novel therapeutic cytomegalovirus DNA vaccine in allogeneic haemopoietic stem-cell transplantation: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Infect Dis. 2012;12(4):290–9.
Heineman TC, Schleiss M, Bernstein DI, Spaete RR, Yan L, Duke G, et al. A phase 1 study of 4 live, recombinant human cytomegalovirus Towne/Toledo chimeric vaccines. J Infect Dis. 2006;193(10):1350–60.
Schleiss MR. Cytomegalovirus in the neonate: immune correlates of infection and protection. Clin Dev Immunol. 2013;2013:501801.
Wussow F, Chiuppesi F, Martinez J, Campo J, Johnson E, Flechsig C, et al. Human cytomegalovirus vaccine based on the envelope gH/gL pentamer complex. PLoS Pathog. 2014;10(11):e1004524.
Zhong J, Khanna R. Delineating the role of CD4+ T cells in the activation of human cytomegalovirus-specific immune responses following immunization with Ad-gBCMVpoly vaccine: implications for vaccination of immunocompromised individuals. J Gen Virol. 2010;91(Pt 12):2994–3001.
Renzette N, Gibson L, Jensen JD, Kowalik TF. Human cytomegalovirus intrahost evolution-a new avenue for understanding and controlling herpesvirus infections. Curr Opin Virol. 2014;8:109–15.
La Rosa C, Diamond DJ. The immune response to human CMV. Futur Virol. 2012;7(3):279–93.
Bohm V, Podlech J, Thomas D, Deegen P, Pahl-Seibert MF, Lemmermann NA, et al. Epitope-specific in vivo protection against cytomegalovirus disease by CD8 T cells in the murine model of preemptive immunotherapy. Med Microbiol Immunol. 2008;197(2):135–44.
Jeitziner SM, Walton SM, Torti N, Oxenius A. Adoptive transfer of cytomegalovirus-specific effector CD4+ T cells provides antiviral protection from murine CMV infection. Eur J Immunol. 2013;43(11):2886–95.
Bunde T, Kirchner A, Hoffmeister B, Habedank D, Hetzer R, Cherepnev G, et al. Protection from cytomegalovirus after transplantation is correlated with immediate early 1-specific CD8 T cells. J Exp Med. 2005;201(7):1031–6.
Gamadia LE, Remmerswaal EB, Weel JF, Bemelman F, van Lier RA, Ten Berge IJ. Primary immune responses to human CMV: a critical role for IFN-gamma-producing CD4+ T cells in protection against CMV disease. Blood. 2003;101(7):2686–92.
Sacre K, Carcelain G, Cassoux N, Fillet AM, Costagliola D, Vittecoq D, et al. Repertoire, diversity, and differentiation of specific CD8 T cells are associated with immune protection against human cytomegalovirus disease. J Exp Med. 2005;201(12):1999–2010.
Luo XH, Huang XJ, Liu KY, Xu LP, Liu DH. Protective immunity transferred by infusion of cytomegalovirus-specific CD8(+) T cells within donor grafts: its associations with cytomegalovirus reactivation following unmanipulated allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2010;16(7):994–1004.
Chen SF, Tu WW, Sharp MA, Tongson EC, He XS, Greenberg HB, et al. Antiviral CD8 T cells in the control of primary human cytomegalovirus infection in early childhood. J Infect Dis. 2004;189(9):1619–27.
Gibson L, Dooley S, Trzmielina S, Somasundaran M, Fisher D, Revello MG, et al. Cytomegalovirus (CMV) IE1- and pp 65-specific CD8+ T cell responses broaden over time after primary CMV infection in infants. J Infect Dis. 2007;195(12):1789–98.
Gibson L, Piccinini G, Lilleri D, Revello MG, Wang Z, Markel S, et al. Human cytomegalovirus proteins pp 65 and immediate early protein 1 are common targets for CD8+ T cell responses in children with congenital or postnatal human cytomegalovirus infection. J Immunol. 2004;172(4):2256–64.
Marchant A, Appay V, Van Der Sande M, Dulphy N, Liesnard C, Kidd M, et al. Mature CD8(+) T lymphocyte response to viral infection during fetal life. J Clin Invest. 2003;111(11):1747–55.
Miles DJ, Sande M, Kaye S, Crozier S, Ojuola O, Palmero MS, et al. CD4(+) T cell responses to cytomegalovirus in early life: a prospective birth cohort study. J Infect Dis. 2008;197(5):658–62.
Tu W, Chen S, Sharp M, Dekker C, Manganello AM, Tongson EC, et al. Persistent and selective deficiency of CD4+ T cell immunity to cytomegalovirus in immunocompetent young children. J Immunol. 2004;172(5):3260–7.
Lidehall AK, Engman ML, Sund F, Malm G, Lewensohn-Fuchs I, Ewald U, et al. Cytomegalovirus-specific CD4 and CD8 T cell responses in infants and children. Scand J Immunol. 2013;77(2):135–43.
Mahnke YD, Brodie TM, Sallusto F, Roederer M, Lugli E. The who’s who of T-cell differentiation: human memory T-cell subsets. Eur J Immunol. 2013;43(11):2797–809.
Betts MR, Nason MC, West SM, De Rosa SC, Migueles SA, Abraham J, et al. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood. 2006;107(12):4781–9.
Nebbia G, Mattes FM, Smith C, Hainsworth E, Kopycinski J, Burroughs A, et al. Polyfunctional cytomegalovirus-specific CD4+ and pp 65 CD8+ T cells protect against high-level replication after liver transplantation. Am J Transplant. 2008;8(12):2590–9.
Zhou W, Longmate J, Lacey SF, Palmer JM, Gallez-Hawkins G, Thao L, et al. Impact of donor CMV status on viral infection and reconstitution of multifunction CMV-specific T cells in CMV-positive transplant recipients. Blood. 2009;113(25):6465–76.
Bernstein DI, Reap EA, Katen K, Watson A, Smith K, Norberg P, et al. Randomized, double-blind, Phase 1 trial of an alphavirus replicon vaccine for cytomegalovirus in CMV seronegative adult volunteers. Vaccine. 2009;28(2):484–93.
Precopio ML, Betts MR, Parrino J, Price DA, Gostick E, Ambrozak DR, et al. Immunization with vaccinia virus induces polyfunctional and phenotypically distinctive CD8(+) T cell responses. J Exp Med. 2007;204(6):1405–16.
Soares AP, Scriba TJ, Joseph S, Harbacheuski R, Murray RA, Gelderbloem SJ, et al. Bacillus Calmette-Guerin vaccination of human newborns induces T cells with complex cytokine and phenotypic profiles. J Immunol. 2008;180(5):3569–77.
Schmueck M, Fischer AM, Hammoud B, Brestrich G, Fuehrer H, Luu SH, et al. Preferential expansion of human virus-specific multifunctional central memory T cells by partial targeting of the IL-2 receptor signaling pathway: the key role of CD4+ T cells. J Immunol. 2012;188(10):5189–98.
Gerna G, Revello MG, Percivalle E, Zavattoni M, Parea M, Battaglia M. Quantification of human cytomegalovirus viremia by using monoclonal antibodies to different viral proteins. J Clin Microbiol. 1990;28(12):2681–8.
Revello MG, Zavattoni M, Baldanti F, Sarasini A, Paolucci S, Gerna G. Diagnostic and prognostic value of human cytomegalovirus load and IgM antibody in blood of congenitally infected newborns. J Clin Virol. 1999;14(1):57–66.
Revello MG, Zavattoni M, Sarasini A, Percivalle E, Simoncini L, Gerna G. Human cytomegalovirus in blood of immunocompetent persons during primary infection: prognostic implications for pregnancy. J Infect Dis. 1998;177(5):1170–5.
Revello MG, Gerna G. Diagnosis and management of human cytomegalovirus infection in the mother, fetus, and newborn infant. Clin Microbiol Rev. 2002;15(4):680–715.
Revello MG, Lilleri D, Zavattoni M, Furione M, Genini E, Comolli G, et al. Lymphoproliferative response in primary human cytomegalovirus (HCMV) infection is delayed in HCMV transmitter mothers. J Infect Dis. 2006;193(2):269–76.
Jones CE, Naidoo S, De Beer C, Esser M, Kampmann B, Hesseling AC. Maternal HIV infection and antibody responses against vaccine-preventable diseases in uninfected infants. JAMA. 2011;305(6):576–84.
Johnson DC, McFarland EJ, Muresan P, Fenton T, McNamara J, Read JS, et al. Safety and immunogenicity of an HIV-1 recombinant canarypox vaccine in newborns and infants of HIV-1-infected women. J Infect Dis. 2005;192(12):2129–33.
Wang Z, Zhou W, Srivastava T, La Rosa C, Mandarino A, Forman SJ, et al. A fusion protein of HCMV IE1 exon4 and IE2 exon5 stimulates potent cellular immunity in an MVA vaccine vector. Virology. 2008;377(2):379–90.
Revello MG, Sarasini A, Zavattoni M, Baldanti F, Gerna G. Improved prenatal diagnosis of congenital human cytomegalovirus infection by a modified nested polymerase chain reaction. J Med Virol. 1998;56(1):99–103.
Gerna G, Vitulo P, Rovida F, Lilleri D, Pellegrini C, Oggionni T, et al. Impact of human metapneumovirus and human cytomegalovirus versus other respiratory viruses on the lower respiratory tract infections of lung transplant recipients. J Med Virol. 2006;78(3):408–16.
Roederer M, Nozzi JL, Nason MC. SPICE: exploration and analysis of post-cytometric complex multivariate datasets. Cytometry A. 2011;79(2):167–74.
Lazzarotto T, Guerra B, Lanari M, Gabrielli L, Landini MP. New advances in the diagnosis of congenital cytomegalovirus infection. J Clin Virol. 2008;41(3):192–7.
Sylwester AW, Mitchell BL, Edgar JB, Taormina C, Pelte C, Ruchti F, et al. Broadly targeted human cytomegalovirus-specific CD4+ and CD8+ T cells dominate the memory compartments of exposed subjects. J Exp Med. 2005;202(5):673–85.
Griffiths P, Plotkin S, Mocarski E, Pass R, Schleiss M, Krause P, et al. Desirability and feasibility of a vaccine against cytomegalovirus. Vaccine. 2013;31 Suppl 2:B197–203.
Azevedo RS, Amaku M. Modelling immunization strategies with cytomegalovirus vaccine candidates. Epidemiol Infect. 2011;139(12):1818–26.
Mansoor N, Abel B, Scriba TJ, Hughes J, de Kock M, Tameris M, et al. Significantly skewed memory CD8+ T cell subsets in HIV-1 infected infants during the first year of life. Clin Immunol. 2009;130(3):280–9.
Schietinger A, Greenberg PD. Tolerance and exhaustion: defining mechanisms of T cell dysfunction. Trends Immunol. 2014;35(2):51–60.
Fulop T, Larbi A, Pawelec G. Human T cell aging and the impact of persistent viral infections. Front Immunol. 2013;4:271.
Bronke C, Jansen CA, Westerlaken GH, De Cuyper IM, Miedema F, Tesselaar K, et al. Shift of CMV-specific CD4+ T-cells to the highly differentiated CD45RO-CD27- phenotype parallels loss of proliferative capacity and precedes progression to HIV-related CMV end-organ disease. Clin Immunol. 2007;124(2):190–9.
Pourgheysari B, Piper KP, McLarnon A, Arrazi J, Bruton R, Clark F, et al. Early reconstitution of effector memory CD4+ CMV-specific T cells protects against CMV reactivation following allogeneic SCT. Bone Marrow Transplant. 2009;43(11):853–61.
Lilleri D, Fornara C, Revello MG, Gerna G. Human cytomegalovirus-specific memory CD8+ and CD4+ T cell differentiation after primary infection. J Infect Dis. 2008;198(4):536–43.
Casazza JP, Betts MR, Price DA, Precopio ML, Ruff LE, Brenchley JM, et al. Acquisition of direct antiviral effector functions by CMV-specific CD4+ T lymphocytes with cellular maturation. J Exp Med. 2006;203(13):2865–77.
Gehrz RC, Marker SC, Knorr SO, Kalis JM, Balfour Jr HH. Specific cell-mediated immune defect in active cytomegalovirus infection of young children and their mothers. Lancet. 1977;2(8043):844–7.
Pass RF, Stagno S, Britt WJ, Alford CA. Specific cell-mediated immunity and the natural history of congenital infection with cytomegalovirus. J Infect Dis. 1983;148(6):953–61.
Starr SE, Tolpin MD, Friedman HM, Paucker K, Plotkin SA. Impaired cellular immunity to cytomegalovirus in congenitally infected children and their mothers. J Infect Dis. 1979;140(4):500–5.
Pass RF, Dworsky ME, Whitley RJ, August AM, Stagno S, Alford Jr CA. Specific lymphocyte blastogenic responses in children with cytomegalovirus and herpes simplex virus infections acquired early in infancy. Infect Immun. 1981;34(1):166–70.
Kim TK, St John LS, Wieder ED, Khalili J, Ma Q, Komanduri KV. Human late memory CD8+ T cells have a distinct cytokine signature characterized by CC chemokine production without IL-2 production. J Immunol. 2009;183(10):6167–74.
Riou C, Treurnicht F, Abrahams MR, Mlisana K, Liu MK, Goonetilleke N, et al. Increased memory differentiation is associated with decreased polyfunctionality for HIV but not for cytomegalovirus-specific CD8+ T cells. J Immunol. 2012;189(8):3838–47.
Thobakgale CF, Streeck H, Mkhwanazi N, Mncube Z, Maphumulo L, Chonco F, et al. Short communication: CD8(+) T cell polyfunctionality profiles in progressive and nonprogressive pediatric HIV type 1 infection. AIDS Res Hum Retroviruses. 2011;27(9):1005–12.
Huang S, Dunkley-Thompson J, Tang Y, Macklin EA, Steel-Duncan J, Singh-Minott I, et al. Deficiency of HIV-Gag-specific T cells in early childhood correlates with poor viral containment. J Immunol. 2008;181(11):8103–11.
Ritz N, Strach M, Yau C, Dutta B, Tebruegge M, Connell TG, et al. A comparative analysis of polyfunctional T cells and secreted cytokines induced by Bacille Calmette-Guerin immunisation in children and adults. PLoS One. 2012;7(7):e37535.
Cannon MJ, Hyde TB, Schmid DS. Review of cytomegalovirus shedding in bodily fluids and relevance to congenital cytomegalovirus infection. Rev Med Virol. 2011;21(4):240–55.
Kimberlin DW, Lin CY, Sanchez PJ, Demmler GJ, Dankner W, Shelton M, et al. Effect of ganciclovir therapy on hearing in symptomatic congenital cytomegalovirus disease involving the central nervous system: a randomized, controlled trial. J Pediatr. 2003;143(1):16–25.
McCarron MJ, Reen DJ. Neonatal CD8+ T-cell differentiation is dependent on interleukin-12. Hum Immunol. 2010;71(12):1172–9.
Debock I, Flamand V. Unbalanced neonatal CD4(+) T-cell immunity. Front Immunol. 2014;5:393.
Sandberg JK, Fast NM, Jordan KA, Furlan SN, Barbour JD, Fennelly G, et al. HIV-specific CD8+ T cell function in children with vertically acquired HIV-1 infection is critically influenced by age and the state of the CD4+ T cell compartment. J Immunol. 2003;170(8):4403–10.
Antoine P, Olislagers V, Huygens A, Lecomte S, Liesnard C, Donner C, et al. Functional exhaustion of CD4+ T lymphocytes during primary cytomegalovirus infection. J Immunol. 2012;189(5):2665–72.
Slavuljica I, Kvestak D, Huszthy PC, Kosmac K, Britt WJ, Jonjic S. Immunobiology of congenital cytomegalovirus infection of the central nervous system-the murine cytomegalovirus model. Cell Mol Immunol. 2014.
Gabrielli L, Bonasoni MP, Santini D, Piccirilli G, Chiereghin A, Petrisli E, et al. Congenital cytomegalovirus infection: patterns of fetal brain damage. Clin Microbiol Infect. 2012;18(10):E419–27.
Khan N, Bruton R, Taylor GS, Cobbold M, Jones TR, Rickinson AB, et al. Identification of cytomegalovirus-specific cytotoxic T lymphocytes in vitro is greatly enhanced by the use of recombinant virus lacking the US2 to US11 region or modified vaccinia virus Ankara expressing individual viral genes. J Virol. 2005;79(5):2869–79.
Acknowledgments
We thank Wanda DePasquale for assistance in preparing the manuscript, Linda Lambrecht for managing the shipping and storage of clinical samples from participating sites, Robin Brody and Thomas Greenough for helpful discussion of flow cytometry, Maripat Toye for specimen collection and administrative support, and Maria Grazia Revello for clinical samples.
Funding
This work is supported by Thrasher Research Fund (to L.G.); the following grants from the National Institutes of Health: K08AI062752 (to L.G.), National Center for Research Resources UL1RR031982 (partial support to C.M.B.), AI063356, AI103960, and CA077544 (partial support to D.J.D.), CA033572 (to The City of Hope Cancer Center), R01HD040450 (K.L.); K24HD001489 (K.L.), and the UMass Center For AIDS Research (AI042845); and Ministero della Salute, Istituto de Ricovero e Cura a Carattere Scientifico Policlinico San Matteo Ricerca Finalizzata, Convenzione 126, and Ricerca Corrente (Grant 80513) (to D.L.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gibson, L., Barysauskas, C.M., McManus, M. et al. Reduced Frequencies of Polyfunctional CMV-Specific T Cell Responses in Infants with Congenital CMV Infection. J Clin Immunol 35, 289–301 (2015). https://doi.org/10.1007/s10875-015-0139-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10875-015-0139-3